Abstract
Carex myosuroides Villars, 1779 is a typical alpine sedge with both ecological and agricultural value. The work reported here is the first complete chloroplast genome of this species. The chloroplast genome, with a total size of 185,609 bp, consists of two inverted repeats (IRs, 38,374 bp) separated by a large single-copy (LSC, 99,911 bp) region, and a small single-copy (SSC, 8950 bp) region. The overall genome GC content is 34.12%. The genome contains 125 genes, consisting of 82 protein-coding genes, 35 tRNA genes, and eight rRNA genes. Phylogenetic analysis supports the taxonomic treatment of incorporating genus Kobresia to a broader circumscription of Carex. Our work could be helpful to future research on Cyperaceae.
Carex myosuroides Villars, 1779, formerly known as Kobresia myosuroides (Villars) Foiri, 1896 (Zhang and Noltie Citation2010), is a typical sedge in alpine environment. It is the only known sedge to harbor associations with ectomycorrhizal fungi, establishing an effective way of transferring amino acid-nitrogen from the soil solution (Lipson et al. Citation1999). Thus, it usually appears as the main species in high-altitude vegetation, especially in highland meadow. It is reported that this species also contributes a notable part to the feeding of livestock in Qinghai-Tibet Plateau (Miehe et al. Citation2019). As alpine ecosystems commonly are considered to be more prone to challenges brought up by climate change, the integrity and vigor of alpine dominant species is of vital importance (Li et al. Citation2020). However, molecular information to date is limited for alpine sedges. Our work here presents the first annotated full-length assembly of the chloroplast of C. myosuroides, which could be helpful to future research on Cyperaceae.
The samples were collected at Dongling mountain, Beijing, China (GPS: E115°30′11″, N 40°3′7″). Fresh leaves were collected and wrapped in drikold, then transferred to be stored at −80 °C lab environment. The relevant specimen was deposited at the herbarium of Beijing Museum of Nature History (http://www.bmnh.org.cn/en/) under the voucher number BJM0271944 (contact [email protected]). The total genomic DNA was extracted using DP305-3 plant genomic DNA Kit (Tiangen, Beijing, China) according to the standard protocol. The sequencing platform was Illumina NovaSeq 6000. Totally, 19,595,642 high-quality clean reads (PE 150) were generated. Aligning, assembly and annotation were conducted by bowtie2 (v2.2.4) (Langmead and Salzberg Citation2012), SPAdes (v3.10.1) (Bankevich et al. Citation2012), SSPACE (v2.0) (Boetzer et al. Citation2011), and Gapfiller (v2.1.1) (Nadalin et al. Citation2012). The assembled and annotated chloroplast genome sequence has been submitted to GenBank under the accession number MZ962720.1.
The plastome of C. myosuroides is a circular DNA molecule with a length of 185,609 bp. Although this genome size is notably higher than the average genome size of monocots (0.144 M) presented by Mohanta et al. (Citation2020), it is comparable to several published Carex chloroplast genomes (∼0.187 M), indicating further investigation is needed for a likely unique evolution trajectory of the sedge group. The full plastome consists of a large single-copy (LSC) region of 99,911 bp, small single-copy (SSC) region of 8950 bp and two inverted repeats (IRs) of 38,374 bp by each. The overall GC content of C. myosuroides chloroplast genome was 34.12%, similar to the average level of 36.82% (Mohanta et al. Citation2020), with corresponding GC values of the LSC, SSC, and IR regions for 32.01%, 26.96%, and 37.69%, respectively. The chloroplast genome of C. myosuroides comprises 125 genes, including 82 protein-coding genes, eight ribosomal RNA (rRNA) genes, and 35 transfer RNA (tRNA) genes.
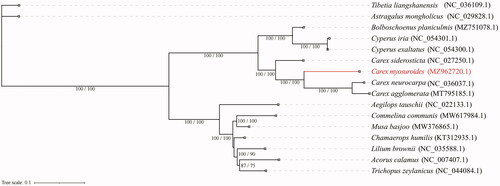
We constructed a maximum likelihood tree to explore the evolutionary position of C. myosuroides (). In total, 16 complete chloroplast genomes were used to build the phylogeny. Two species from Fabaceae were used as the outgroup (Astragalus mongholicus Bunge, 1868 and Tibetia liangshanensis Li, Pei Chun, 1981). The sequences were aligned using the default settings in MAFFT v7 (Katoh et al. Citation2019). The phylogenetic analyses were conducted using IQTREE v1.6.7 (Nguyen et al. Citation2015). The fitted model was TVM + F+R3, with branch support tested by SH-aLRT (1000 replicates) and ultrafast bootstrap (1000 replicates). Branches were labeled if their SH-aLRT score >85% and ultrafast bootstrap support >75%. Based on the current sampling extent, our result presents a phylogeny with most internal relationships well supported. This result is also in accordance with the taxonomic treatment of incorporating genus Kobresia to a broader circumscription of Carex (Global Carex Group Citation2015). Our work could be helpful to further researches in Cyperaceae.
Authors contributions
H-Y Chen and X-F Xia conceived and designed the experiment. Y Ning and X-F Xia collected the specimen. Z Pan carried out the extraction and sequencing. Y Ning analyzed the data and made the figure. H-Y Chen and X-F Xia contributed to writing and editing the manuscript. Y Ning and Z Pan revised the manuscript.
Acknowledgements
Ethical statement: This study was approved by the Committee of Beijing Museum of Nature History. All the procedures of the field and lab work are in accordance with the local/international guidelines. No protected/endangered species was involved.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in the GenBank database at https://www.ncbi.nlm.nih.gov/, under accession number [MZ962720.1]. The associated BioProject, SRA, and BioSample numbers are PRJNA772905, SRR 16494724, and SAMN 22419664, respectively.
Additional information
Funding
References
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et. al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19(5):455–477.
- Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W. 2011. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics. 27(4):578–579.
- Global Carex Group. 2015. Making Carex monophyletic (Cyperaceae, tribe Cariceae): a new broader circumscription. Bot J Linn Soc. 179(1):1–42.
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 20(4):1160–1166.
- Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods. 9(4):357–359.
- Li M, Zhang X, He Y, Niu B, Wu J. 2020. Assessment of the vulnerability of alpine grasslands on the Qinghai-Tibetan Plateau. Peer J. 8:e8513.
- Lipson DA, Schadt CW, Schmidt SK, Monson RK. 1999. Ectomycorrhizal transfer of amino acid‐nitrogen to the alpine sedge Kobresia myosuroides. New Phytol. 142(1):163–167.
- Miehe G, Schleuss P-M, Seeber E, Babel W, Biermann T, Braendle M, Chen F, Coners H, Foken T, Gerken T, et al. 2019. The Kobresia pygmaea ecosystem of the Tibetan highlands – origin, functioning and degradation of the world's largest pastoral alpine ecosystem: Kobresia pastures of Tibet. Sci Total Environ. 648:754–771.
- Mohanta TK, Mishra AK, Khan A, Hashem A, Abd_Allah EF, Al-Harrasi A. 2020. Gene loss and evolution of the plastome. Genes. 11(10):1133.
- Nadalin F, Vezzi F, Policriti A. 2012. GapFiller: a de novo assembly approach to fill the gap within paired reads. BMC Bioinformatics. 13:1–16.
- Nguyen L, Schmidt HA, Von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274.
- Zhang SR, Noltie HJ. 2010. Kobresia Willdenow. In: Wu ZY, Raven PH, editors. Flora of China. Vol. 23. St. Louis: Missouri Botanical Garden Press; p. 269–285.