Abstract
In this study, we analyzed the complete sequence of the chloroplast genome of Chrysanthemum rupestre Matsum. et Koidz., 1910, a diploid disciform capitula species of Chrysanthemum endemic to Japan, formerly classified as Ajania rupestris (Matsum. & Koidz.) Muldashev, Bot. Zhurn. (Moscow & Leningrad), 1983. The chloroplast genome of C. rupestre has a typical conserved quadripartite structure of 151,061 bp in length, comprising a large single-copy region (82,846 bp), a small single-copy region (18,301 bp), and a pair of inverted repeat regions (each 24,957 bp). Phylogenetic analysis indicated that C. rupestre clustered with other Chrysanthemum species, including another former Ajania species, Chrysanthemum pacificum Nakai, 1928. However, Ajania variifolia (C.C.Chang) Tzvelev, 1961, which is a synonym of Phaeostigma variifolium (C.C.Chang) Muldashev, 1981, was placed outside the Chrysanthemum clade, thereby implying that the former genus Ajania includes heterogeneous species.
The genus Chrysanthemum, belonging to the tribe Anthemideae within the family Asteraceae, includes cultivated chrysanthemum (Chrysanthemum morifolium Ramat., 1792), which is among the most important ornamental flowers (Bremer and Humphries Citation1993; Ohashi and Yonekura Citation2004). Chrysanthemum species have been classified into four groups, the Indicum, Makinoi, Zawadskii, and Ajania groups, based on their morphological characteristics (Tanaka and Shimotomai Citation1978). Species in the Ajania group were formerly classified into the genus Ajania, which is characterized by a disciform capitulum. However, molecular phylogenetic analyses have revealed that at least some of the species within the former Ajania genus form a cluster with the Chrysanthemum genus (Masuda et al. Citation2009; Liu et al. Citation2012; Nakano et al. Citation2019). To investigate the relationship between the genera Chrysanthemum and the former Ajania, we determined the sequence of the whole chloroplast genome of Chrysanthemum rupestre, a diploid species belonging to the Ajania group endemic to Japan.
The C. rupestre plant (AME15) used for sequencing was collected at Tomi, Japan (N36°21′32.4′′, E138°19′51.5994′′) under permission from Ministry of the Environment of Japan and Tomi city, and is available from National Bioresource Project Chrysanthemum (https://shigen.nig.ac.jp/chrysanthemum/). A voucher specimen (SH1001) and extracted DNA have been deposited in the Herbarium of the Laboratory of Plant Chromosome and Gene Stock, Hiroshima University (contact M. Kusaba, [email protected]). Total genomic DNA was extracted from fresh leaves of C. rupestre using a modified CTAB procedure (Doyle and Doyle Citation1987). The sequence of the whole chloroplast genome was determined by assembling whole-genome shotgun sequences obtained by using a Novaseq 6000 (Illumina) sequencer in conjunction with GetOrganelle v1.7.4.1 software (Jin et al. Citation2020). The fastq file extracted using GetOrganelle was submitted to the Genbank SRA database (PRJNA782395). Sequence analysis using the MPI-MP CHLOROBOX GeSeq program (Tillich et al. Citation2017) revealed that the complete chloroplast genome of C. rupestre has a typical conserved quadripartite structure of 151,061 bp in length with an overall GC content of 37.47%, comprising a large single-copy region (LSC, 82,846 bp), a small single-copy region (SSC, 18,301 bp), and a pair of inverted repeat regions (IR, each 24,957 bp), respectively, which are similar to those of another Chrysanthmum species, C. morifolium cv. Orizaba (LSC, 82,858 bp; SSC, 18,294 bp; IR, each 24,954 bp; and GC content 37.45%; Xia et al. Citation2021). The chloroplast genome contains 131 genes, comprising 87 protein-coding genes, 8 rRNA genes, and 36 tRNA genes.
Phylogenetic analysis was carried out using whole chloroplast genome sequences of 21 Anthemideae species (). Having adjusted the SSC direction, the dataset was aligned using MAFFT v7.490 (Katoh and Standley Citation2013). The phylogenetic tree was constructed by the maximum likelihood method using PhyML v3.0 with 1000 bootstrap replicates (Guindon et al. Citation2010). The phylogenetic tree indicated that the genus Chrysanthemum forms a single clade with high bootstrap support and a neighboring genus Artemisia clade.
Figure 1. A phylogenetic tree of Chrysanthemum rupestre and related species based on complete chloroplast genome sequences. The phylogenetic tree was constructed using maximum-likelihood method with 1000 bootstrap replicates. Names of species and GenBank accession numbers are shown. Chrysanthemum repestre is boxed. The bootstrap support values are shown at the branches. Our analysis suggests that Opisthopappus taihangensis (Y. Ling) C. Shih,1979, which is synonym to Chrysanthemum taihangens Y. Ling, 1939, belongs to the genus Chrysanthemum. In our analysis, Crossostephium chinense was not clustered with the Artemisia species unlike in the previous report (Chen et al. Citation2019).
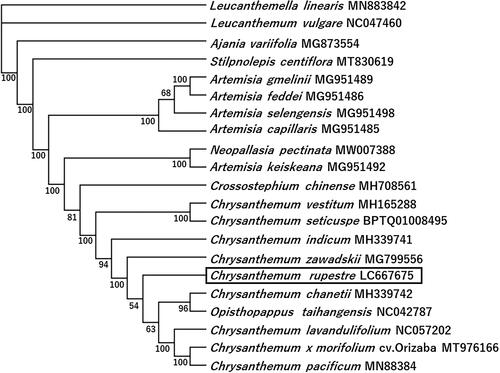
The former Ajania species C. rupestre and Chrysanthemum pacificum are included in the Chrysanthemum clade, whereas Ajania variifolia (syn. Phaeostigma variifolium) is placed outside the Chrysanthemum clade. However, Masuda et al. (Citation2009) reported that P. variifolium is included in the Chrysanthemum clade and distantly related to Phaeostigma salicifolium (Mattf.) Muldashev, 1981, implying that the taxonomic position of P. variifolium remains to be determined. Nevertheless, our findings indicate that the former genus Ajania includes heterogeneous species, comprising at least two distinct groups, namely, P. variifolium and C. rupestre/C. pacificum.
Furthermore, on the basis of phylogenetic analysis using nuclear-encoded genes, Shen et al. (Citation2021) revealed that C. pacificum, Chrysanthemum shiwogiku Kitam., 1935 (syn. Ajania shiwogiku (Kitam.) Bremer and Humphries, Citation1993), and Chrysanthemum pallasianum Kom., 1907 (syn. A. pallasiana (Fisch. ex Besser) Poljakov, 1955), which are endemic to Japan, fall within the Chrysanthemum clade, although other Ajania species form a clade separate from the Chrysanthemum clade. It was accordingly inferred that the genomes of C. pacificum, C. shiwogiku, and C. pallasianum have been influenced by the introgression of the genome of Indicum complex (Indicum and Makinoi groups) during their evolution. However, molecular phylogenetic analysis based on a nuclear-encoded gene has indicated that C. rupestre, a diploid Ajania group species endemic to Japan, forms a clade with Chrysanthemum potaninii (Krasch.) Hand.-Mazz., 1938 and Chrysanthemum nematolobum Hand.-Mazz.,1938, which are diploid Ajania group species endemic to China (Nakano et al. Citation2019). Therefore, we anticipate analyses of the whole genome sequences of C. rupestre in addition to Chrysanthemum seticuspe (Maxim.) Hand.-Mazz., 1936 and Chrysanthemum makinoi Matsum. et Nakai, 1916 (Nakano et al. Citation2021; van Lieshout et al. Citation2021) may provide a number of clues to clarify the evolutionary history of the Ajania group species.
Authors’ contributions
Y.M., M.N., and M.K. conceived the experiments and designed the project. Y.M. performed phylogenetic analysis. M.N. conducted the genomic sequence analysis Y.M. and M.K. wrote the manuscript. All authors agree to be accountable for all aspects of the work.
Acknowledgments
We thank Kenji Taniguchi for a meaningful discussion.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at [https://www.ncbi.nlm.nih.gov] under the accession no. LC667675. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA782395, SRR16996951, and SAMN23377311 respectively.
Additional information
Funding
References
- Bremer K, Humphries CJ. 1993. Genetic monograph of Asteraceae-Anthemideae. Bull Nat Hist Mus Lond. 23(2):71–177.
- Chen T, Du Y, Zhao H, Dong M, Liu L. 2019. The complete chloroplast genome of Crossostephium chinense (Asteraceae), using genome skimming data. Mitochondrial DNA Part B. 4(1):322–323.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15.
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 59(3):307–321.
- Jin JJ, Yu WB, Yang JB, Song Y, dePamphilis CW, Yi TS, Li DZ. 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21(1):241.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Liu PL, Wan Q, Guo YP, Yang J, Rao GY. 2012. Phylogeny of the genus Chrysanthemum L.: evidence from single-copy nuclear gene and chloroplast DNA sequences. PLoS One. 7(11):e48970.
- Masuda Y, Yukawa T, Kondo K. 2009. Molecular phylogenetic analysis of members of Chrysanthemum and its related genera in the tribe Anthemideae, the Asteraceae in East Asia on the basis of the internal transcribed spacer (ITS) region and the external transcribed spacer (ETS) region of nrDNA. Chromosome Botany. 4(2):25–36.
- Nakano M, Hirakawa H, Fukai E, Toyoda A, Kajitani R, Minakuchi Y, Itoh T, Higuchi Y, Kozuka T, Bono H, et al. 2021. A chromosome-level genome sequence of Chrysanthemum seticuspe, a model species for hexaploid cultivated chrysanthemum. Commun Biol. 4(1):1167.
- Nakano M, Taniguchi K, Masuda Y, Kozuka T, Aruga Y, Han J, Motohara K, Nakata M, Sumitomo K, Hisamatsu T, et al. 2019. A pure line derived from a self-compatible Chrysanthemum seticuspe mutant as a model strain in the genus Chrysanthemum. Plant Sci. 287:110174.
- Ohashi H, Yonekura K. 2004. New combinations in Chrysanthemum (Compositae-Anthemideae) of Asia with a list of Japanese species. J Jpn Bot. 79(3):186–195.
- Shen CZ, Zhang CJ, Chen J, Guo YP. 2021. Clarifying recent adaptive diversification of the chrysanthemum-group on the basis of an updated multilocus phylogeny of subtribe Artemisiinae (Asteraceae: Anthemideae). Front Plant Sci. 12:648026.
- Tanaka R, Shimotomai N. 1978. Nihon-san yasei-giku no shurui [Wild chrysanthemum species in Japan]. Nat Plants. 12:6–11. (Japanese)
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq - versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45(W1):W6–W11.
- van Lieshout N, van Kaauwen M, Kodde L, Arens P, Smulders MJM, Visser RGF, Finkers R. 2021. De novo whole-genome assembly of Chrysanthemum makinoi, a key wild chrysanthemum. G3. 12(1):jkab358.
- Xia H, Xu Y, Zhang J, Huang Z, Luo H, Ye Z, Zhou H. 2021. Complete chloroplast genome sequence of a Dutch cultivar of Chrysanthemum, Chrysanthemum morifolium ‘Orizaba’ (Asteraceae). Mitochondrial DNA Part B. 6(7):1937–1938.
