Abstract
Cipangopaludina ampullacea (Küster, 1852) is a freshwater snail endemic to China. In this study, the complete mitochondrial genome of C. ampullacea was sequenced using next-generation sequencing. The mitogenome is 16,892 bp long and comprises a total of 37 genes, including 13 protein-coding genes, two rRNA genes, and 22 tRNA genes. It is consistent with the basic characteristics of other known viviparid mitochondrial genomes. Phylogenetic analysis using related species mitogenomes showed that Cipangopaludina and Margarya are mutually non monophyletic. Our study provides valuable information to reconstruct the taxonomy and evolution of viviparid snails more comprehensively.
Viviparidae is a family of rather large freshwater gastropods, containing approximately 150 species (Franke et al. Citation2007). Except for South America and Antarctica, this family is represented on every continent, where it inhabits areas such as lakes, ponds, and rivers (Strong et al. Citation2008). Due to the incongruence among the morphology and molecular phylogeny of the Viviparidae, the classification of this family is still ambiguous, especially within the genus Cipangopaludina (Wang et al. Citation2017; Stelbrink et al. Citation2020). Cipangopaludina ampullacea (Küster 1852) is a viviparid snail endemic to China, mainly distributed in Yunnan and Sichuan provinces (Liu et al. Citation1995). Due to the decreasing of its natural populations, C. ampullacea is currently listed as Vulnerable (VU) by the Chinese Species Red List (Vol. III Invertebrates). Studies about the shell morphology of C. ampullaceal are still scarce (Liu et al. Citation1995; Lu et al. Citation2014) and only very few mitochondrial genomes from the genus Cipangopaludina have been published so far (Wang et al. Citation2017; Nasu et al. Citation2020). In this study, we sequenced the complete mitochondrial genome of C. ampullacea as a resource for promoting phylogenetic studies of the Viviparidae. Our study provides valuable data that can be used in the taxonomy and evolution of viviparid snails.
Specimens of C. ampullacea were collected from Lake Dianchi (24°47′11″N, 102°36'52″E), Yunnan, China. Total genomic DNA was extracted using EZNA Mollusk DNA Kit (Omega Bio-Tek, USA) according to the manufacturer’s instructions. The muscle tissue was preserved at −80 °C, and the voucher specimen (number: 315-VIVI-QFNU; contact Guang-Long Xie: [email protected]) was deposited in the Zoology Museum of Qufu Normal University. The mitochondrial genome of C. ampullacea was sequenced on the Illumina Novaseq 6000 sequencing platform with PE150 strategy. The sequence was assembled and mapped within CLC Genomic Workbench 12.0.2 (Qiagen). The mitochondrial genome was annotated using the MITOS webserver (Bernt et al. Citation2013). The preliminary results were compared with the protein-coding genes (PCGs) and rRNA genes of other viviparid species by using BLAST searches. MITOS and ARWEN were used to detect tRNA genes (Laslett and Canback Citation2008). Phylogenetic relationships of Viviparidae were reconstructed by performing Maximum Likelihood (ML) and Bayesian inference (BI). For Maximum Likelihood analyses were inferred by using IQ-Tree, nodal support of the best tree was estimated by performing 10,000 ultrafast bootstrap replicates (Nguyen et al. Citation2015). For the Bayesian analyses were inferred by MrBayes version 3.2.6 (Ronquist et al. Citation2012). Bayesian posterior probabilities of phylogenetic trees were estimated by running four separate runs of each 10 million generations. Each run had four chains, of which one was heated. Sampling rate was every 1000 generations.
The complete mitogenome of C. ampullacea (GenBank accession number: MZ488942) was 16,891 bp in length. Identical to other Viviparidae species, it contains 13 protein-coding genes (PCGs), 22 transfer RNA (tRNAs), and two ribosomal RNA (rRNAs). The base composition of the whole heavy strand is A 27.2%, C 8.2%, G 20.1%, T 44.5%. The A + T content (71.7%) was distinctly higher than the GC content (28.3%). Thirty genes were located on the heavy strand while all others were located on the light strand (seven tRNA genes: trnY, trnC, trnW, trnM, trnQ, trnG, and trnE,). The gene arrangement of C. ampullacea differed slightly from that of other known viviparid mitogenomes, in the translocation on the relative position of the YCWMQGE tRNA clusters (Wang et al. Citation2017).
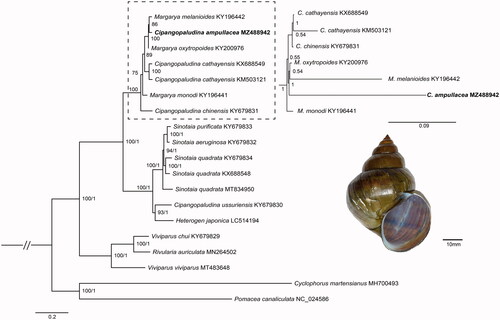
Together with 17 other viviparid species, phylogenetic analysis based on 13 PCGs genes showed that the phylogenetic status of C. ampullacea differed in the BI and ML trees (). In the ML tree, C. ampullacea was the sister group with Margarya melanioides. By contrast, in the BI tree, the C. ampullacea was the sister group with M. oxytropoides + M. melanioides. Moreover, Cipangopaludina and Margarya were non-monophyletic, which is consistent with previous study. The phylogenetic relationships of Cipangopaludina and Margarya were controversial (Wang et al. Citation2017; Stelbrink et al. Citation2020). Hence, these genera need integrative taxonomic revisions.
Ethical approval
The handling of freshwater snails was conducted in accordance with the guidelines on the care and use of animals for scientific purposes set by the Institutional Animal Care and Use Committee (IACUC) of Qufu Normal University, Shandong, China.
Author contributions
M.M.H. and G.L.X. designed the study. M.M.H. and H.Z.C. performed the laboratory work. M.M. H. analyzed the data, prepared figures, and tables, and wrote the paper. L.L.L. supervised the molecular analyses. G.L.X. revised the manuscript. All authors contributed to the critical review and revision of the manuscript.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under accession no MZ488942. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA770541, SRR16304169, and SAMN22224088 respectively.
Additional information
Funding
References
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Franke H, Riedel F, Glaubrecht M, Köhler F, von Rintelen T. 2007. Evolution and biogeography of Southeast Asian viviparids (Gastropoda: Caenogastropoda). In: Jordaens K, Van Houtte N, Van Goethem J, Backeljau T., editors. World Congress of Malacology, Antwerp, Belgium.
- Laslett D, Canback B. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24(2):172–175.
- Liu YY, Zhang WZ, Wang YX. 1995. Distribution of the Viviparidae from China (Mollusk: Gastropoda). Trans Chin Soc Malacol. 5(6):8–16.
- Lu HF, Du LN, Li ZQ, Chen XY, Yang JX. 2014. Morphological analysis of the Chinese Cipangopaludina species (Gastropoda; Caenogastropoda: Viviparidae). Dongwuxue Yanjiu. 35(6):510–527.
- Nasu K, Yokoyama Y, Sun Y, Suzuki-Matsubara M, Teramoto T, Moriyama A, Kawase M, Kumazawa Y. 2020. Mitochondrial genome of Cipangopaludina japonica (Gastropoda: Viviparidae) with a tRNA gene rearrangement. Mitochondrial DNA B. 5(2):1340–1341.
- Nguyen L, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fastand effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542.
- Stelbrink B, Richter R, Köhler F, Riedel F, Strong EE, Van Bocxlaer B, Albrecht C, Hauffe T, Page TJ, Aldridge DC, et al. 2020. Global diversification dynamics since the Jurassic: low dispersal and habitat-dependent evolution explain hotspots of diversity and shell disparity in river snails (Viviparidae). Syst Biol. 69(5):944–961.
- Strong EE, Gargominy O, Ponder WF, Bouchet P. 2008. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia. 595(1):149–166.
- Wang JG, Zhang D, Jakovlic I, Wang WM. 2017. Sequencing of the complete mitochondrial genomes of eight freshwater snail species exposes pervasive paraphyly within the Viviparidae family (Caenogastropoda). PLoS One. 12(7):e0181699.