Abstract
Salix wilhelmsiana M.B. Bieberstein 1819 is a perennial woody plant with high economic and ecological value. In this study, we annotated the chloroplast (cp) genome of Salix wilhelmsiana M.B. The results showed that the length of the complete cp genome is 155,577 bp, which is typically composed of two single-copy regions (large single-copy (LSC) of 84,439 bp and small single-copy (SSC) of 16,221 bp) and a pair of IR regions of 27,457 bp with a quadripartite structure. The genome contains 129 genes, including 84 protein-coding genes, 37 tRNA genes, and eight rRNA genes. The GC content was 36.70%. Phylogenetic analysis based on cp genome sequences of 19 species from the Salicaceae family revealed that S. wilhelmsiana M.B. is closely related to S. viminalis var. gmelinii.
The Salix genus, comprised of trees known for their world-renowned landscape greening and economic value, contains approximately 400 natural species distributed worldwide (Zhou et al. Citation2021). There are approximately 200 species found across provinces in China. Salix wilhelmsiana M.B., one of the most important species of the Salix genus, is a shrub or small tree and is commonly used for its ornamental leaves. It is mostly grown in desert and semidesert areas in northern China and used for its drought-tolerant attributes. However, molecular breeding programs and germplasm resources for S. wilhelmsiana M.B. have been limited due to a lack of genome data. The objectives of our study were to sequence and to assemble the complete chloroplast (cp) genome of S. wilhelmsiana M.B. using next-generation sequencing. Having the complete cp genome of S. wilhelmsiana M.B. elucidated will provide data for determining the phylogeny of the Salix genus and advancing research on S. wilhelmsiana M.B.
Fresh leaves of Salix wilhelmsiana M.B. were collected in Yanchi County in the Ningxia Hui Autonomous Region (China, N37°46′55.82″, E107°24′9.33″). Leaf specimens (No. YCXYL2018003) were deposited in Room 60708 Biotechnology Building, Nanjing Forestry University, Nanjing, China (Li Xiaoping, [email protected]). Total genomic DNA was extracted using the improved CTAB method (Doyle and Doyle Citation1987). The Illumina HiSeq 2000 platform was used for sequencing after the sequencing library was constructed. Approximately, 6.1 GB of clean data were generated after filtering raw data with Fastp (Chen et al. Citation2018). Then, the clean data were used to assemble the complete cp genome using NOVOPlasty software version 4.1 (https://github.com/ndierckx/NOVOPlasty) (Dierckxsens et al. Citation2017). The complete cp genome of Salix koriyanagi was selected from the NCBI database as the reference sequence. CPGAVAS2 software was used for cp genome annotation combined with manual correction (Shi et al. Citation2019). The complete cp genome sequence of S. wilhelmsiana M.B. was submitted to GenBank with the accession number OL405086.
Like to most other angiosperms, the cp of S. wilhelmsiana M.B. is a typical quadripartite structure 155,577 bp in length, including a large single-copy (LSC) of 84,439 bp and a small single-copy (SSC) of 16,221 bp, reversed by two 27,457 bp inverted repeat sequences (IRs). The genome contains 129 genes, including 84 protein-coding genes, 37 tRNA genes, and eight rRNA genes. The total GC content of the circular DNA molecule was 36.70%. The GC content of the LSC was 34.43%, the SSC was 30.98%, and the IRs (IRA and IRB) were each 41.87% ().
Figure 1. Phylogenetic relationships of S. wilhelmsiana M.B. and the other 19 species based on the chloroplast genome sequences, and Arabidopsis thaliana was used as an outgroup.
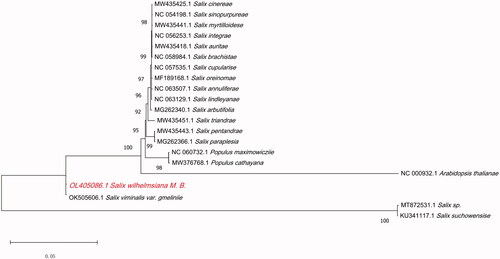
To reveal the relationship between S. wilhelmsiana M.B. and other species in the Salicaceae family, we conducted phylogenetic analyses. The cp genome sequences of 19 published Salicaceae species in the NCBI database, including seventeen Salix and two Populus species, were downloaded. Arabidopsis thaliana was used as an outgroup. All sequences were aligned using the program MAFFTv.7.149 (https://mafft.cbrc.jp/alignment/software/) (Katoh and Standley Citation2013), and a maximum-likelihood phylogenetic tree was constructed by MEGA 11 (Kumar et al. Citation2016) with 1000 bootstrap replicates. We used the Tamura–Nei model, the number of threads was 7, and we used a phylogram type of phylogenetic tree. The results showed that Salix viminalis var. gmelinii is more closely related and sister to a highly supported clade composed of 19 species (Salix cinerea, Salix sinopurpurea, Salix myrtilloides, Salix integra, Salix aurita, Salix brachista, Salix cupularis, Salix oreinoma, Salix annulifera, Salix lindleyana, Salix arbutifolia, Salix triandra, Salix pentandra, Salix paraplesia, Populus maximowiczii, Populus cathayana, Salix viminalis var. gmelinii, Salix sp., and Salix suchowensis). Our research lays a solid foundation for advanced studies on the genetic diversity and phylogeny of S. wilhelmsiana M.B.
Author contributions
Xiaoping Li contributed to the conception of the study; Zhengxuan Wang, Zicheng Yu, Xu Yao, and Jing Wang, performed the experiment; Zhengxuan Wang contributed significantly to analysis and manuscript preparation; Zhengxuan Wang performed the data analyses and wrote the manuscript; Huijie Tang assisted in the collection of willow specimens and some data processing; Xiaoping Li helped perform the analysis with constructive discussions and revised it critically for intellectual content. All authors approve the version to be published and agree to be accountable for all aspects of the work.
Acknowledgements
Regulation statement: This study strictly follows the wild plant protection regulations of the People’s Republic of China, which Promulgated by Decree No. 204 of The State Council of the People’s Republic of China on 30 September 1996, and amended in accordance with Decree No. 687 of The State Council of the People’s Republic of China on 7 October 2017, “Decision of The State Council on Revising Some Administrative Regulations”.
Disclosure statement
No potential conflicts of interest were reported by the author(s).
Data availability statement
The complete chloroplast genome sequence data of S. wilhelmsiana M.B. supporting the findings of this study are openly available in GenBank of NCBI at http://www.ncbi.nlm.nih.gov/ under the accession number OL405086. The associated BioProject, SRA, and BioSample numbers are PRJNA778430, SRR16916512, and SAMN22959552, respectively.
Additional information
Funding
References
- Chen S, Zhou Y, Chen Y, Jia G. 2018. Fastp: an ultrafast all-in-one fastq preprocessor. Bioinformatics. 34(17):i884–i890.
- Dierckxsens N, Mardulyn P, Smits G. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45(4):1–9.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15.
- Katoh K, Standley D. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33(7):1870–1874.
- Shi L, Chen H, Jiang M, Wang L, Wu X, Huang L, Liu C. 2019. CPGAVAS2, an integrated plastome sequence annotator and analyser. Nucleic Acids Res. 47(W1):W65–W73.
- Zhou J, Jiao Z, Guo J, Wang BS, Zheng J. 2021. Complete chloroplast genome sequencing of five Salix species and its application in the phylogeny and taxonomy of the genus. Mitochondrial DNA B Resour. 6(8):2348–2352.
