Abstract
The complete chloroplast (cp) genome of Sparganium angustifolium was sequenced and annotated in the present study. The circular genome is 161,720 bp in length and exhibits a typical quadripartite structure with a large single-copy (LSC, 88,981 bp) and small single-copy (SSC, 18,731 bp) regions, separated by a pair of inverted repeats (IRs, 27,004 bp). The cp genome contains 114 unique genes, including 80 protein-coding, 30 tRNA, and four rRNA genes. The phylogenetic analysis of Typhaceae strongly supported the monophyly of Sparganium and resolved two clades that represented newly revised two subgenera. S. angustifolia has the closest relationship with S. emersum in the present sampling extent.
Sparganium (Typhaceae) is a marsh or aquatic genus including approximately 14 species distributed mainly in temperate and cool regions (Cook and Nicholls Citation1986, Citation1987; Kaul Citation2000). Species of Sparganium are ecologically important in aquatic communities by providing shelter and food for waterfowl and mammals (Fassett Citation1940). S. angustifolium Michaux 1803 is characterized by its long floating stems and leaves (>20 cm in length) different from its congeners (Sun and Simpson Citation2010), and shows a circumboreal distribution at high elevations. To date, the complete chloroplast (cp) genomes of five Sparganium species, S. eurycarpum subsp. coreanum (H.Lev.) C.D.K.Cook & M.S.Nicholls 1867, S. stoloniferum (Buch.-Ham. ex Graebn.) Buch.-Ham. ex Juz. 1934, S. fallax Graebn. 1900, S. glomeratum (Laest. ex Beurl.) Beurl. 1853 and S. stoloniferum subsp. choui (D.Yu) K.Sun 1992 have been reported (Gil et al. Citation2019; Su et al. Citation2019; Huang et al. Citation2021; Lu et al. Citation2021; Zhang et al. Citation2021). As genus Sparganium species are of ecological, phylogenetic and evolutionary value, more genetics resources would facilitate further study on them (Sulman et al. Citation2013). Here, we first reported the complete cp genome of S. angustifolium and reconstructed phylogenetic relationships within Sparganium and test the monophyly of this genus.
Fresh leaves of S. angustifolium were sampled from Hunchun city (130°48.480′E, 42°55.692′N) in Jilin Province of China, and dried with silica gel. The specimen was deposited in Herbarium of the Wuhan University (www.whu.edu.cn, Xinwei Xu, [email protected]) under the voucher number Xu3837. The plant material does not involve ethical conflicts. All the collection and following sequencing work was strictly executed under local legislation and related laboratory regulations to protect wild resources. Total genomic DNA was extracted using the DNA Plantzol Reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol. Library preparation and genomic DNA sequencing on the BGISEQ-500 platform were conducted by the Beijing Genomics Institute (BGI; Shenzhen, China). The obtained paired-end reads were used to assemble the cp genomes using NOVOPlasty4.2 (Dierckxsens et al. Citation2017), with a subunit of the photosystem II (psbA) gene from S. stoloniferum (GenBank accession no. NC_044634) as the seed. The cp genome annotation was performed in program Geneious Prime v2020.0.5 (Kearse et al. Citation2012), with the cp genome of S. stoloniferum as the reference. The start and stop codon positions and the boundaries between introns and exons were manually corrected where necessary. The annotated complete cp genomes of S. angustifolium were submitted to GenBank under the accession no. MZ981724.
The cp genome of S. angustifolium is a circular DNA molecule of 161,720 bp in length. The cp genome has a typical quadripartite structure, consisting of a pair of inverted repeats (IRa and IRb, 27,004 bp) separated by a large single-copy (LSC, 88,981 bp) region and a small single-copy (SSC, 18,731 bp) region. The overall GC content is 36.9%. The IR regions have a higher GC content (42.4%) than the LSC (34.8%) and SSC (31.0%). The cp genome encodes a set of 114 unique genes, including 80 protein-coding, 30 tRNA, and four rRNA genes. Among the genes identified, nine genes (atpF, ndhA, ndhB, petB, petD, rpl2, rpl16, rpoC1, and rps16) contain one intron and three genes (clpP, rps12, and ycf3) comprise of two introns. Twenty genes (eight protein-coding genes, eight tRNA genes, and four rRNA genes) are duplicated in the IR region, and ycf1 is pseudogene.
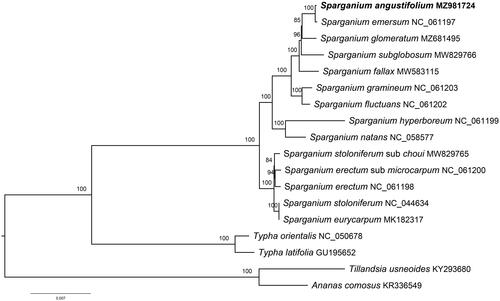
Chloroplast genomes of 15 Typhaceae species and two Bromeliaceae species were downloaded from GenBank for the phylogenetic analysis. A total of 18 sequences were aligned using the default settings in MAFFT v7.3 (Katoh and Standley Citation2013). The maximum-likelihood tree was constructed using IQTREE v1.6.7 with 1000 bootstrap replicates (Nguyen et al. Citation2015). Ananas comosus (L.) Merr. 1917 and Tillandsia usneoides (L.) L. 1762 were set as outgroups. The phylogenetic analysis provided strong support for monophyly of Sparganium (100% BS) and two main clades were recovered, which represented two Sparganium subgenera proposed by Sulman et al. (Citation2013). In the present sampling extent, S. angustifolia has the closest relationship with S. emersum ().
Author contributions
QL and ZY conceived the idea; QL contributed to the sampling; XC, JL, and JY collected the data; WM analyzed the data. The manuscript was written by ZY and WM. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/ under the accession no. MZ981724. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA750266, SRR16530833, and SAMN20691739, respectively.
Additional information
Funding
References
- Cook CDK, Nicholls MS. 1986. A monographic study of the genus Sparganium (Sparganiaceae). Part 1, subgenus Xanthosparganium Holmberg. Bot Helv. 96:213–267.
- Cook CDK, Nicholls MS. 1987. A monographic study of the genus Sparganium (Sparganiaceae). Part 2, subgenus Sparganium. Bot Helv. 97:1–44.
- Dierckxsens N, Mardulyn P, Smits G. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45(4):e18.
- Fassett NC. 1940. A manual of aquatic plants. Madison: University of Wisconsin Press.
- Gil HY, Ha YH, Choi KS, Lee JS, Chang KS, Choi K. 2019. The chloroplast genome sequence of an aquatic plant, Sparganium eurycarpum subsp. coreanum (Typhaceae). Mitochondrial DNA Part B. 4(1):684–685.
- Huang L, Zhang QY, Xu XW. 2021. Characterization of the complete chloroplast genome of an aquatic plant, Sparganium stoloniferum subsp. choui (Typhaceae). Mitochondrial DNA Part B. 6(10):3078–3079.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649.
- Kaul RB. 2000. Sparganiaceae. In: Flora of North America North of Mexico. Vol. 22: Magnoliophyta: Alismatidae, Arecidae, Commelinidae (in Part) and Zingiberidae. New York and Oxford: Oxford University Press; p. 270–277.
- Lu QX, Ren LJ, Wang YN, Xiao Y, Li YD, Ying JN, Zhang Y, Wang R, Qi ZC, You ZY. 2021. Characterization of the complete chloroplast genome of Sparganium glomeratum (Typhaceae) from Jilin Province, China and phylogenetic analysis. Mitochondrial DNA B Resour. 6(11):3253–3254.
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274.
- Sulman JD, Drew BT, Drummond C, Hayasaka E, Sytsma KJ. 2013. Systematics, biogeography, and character evolution of Sparganium (Typhaceae): diversification of a widespread, aquatic lineage. Am J Bot. 100(10):2023–2039.
- Sun K, Simpson DA. 2010. Typhaceae. In: Wu ZY, Raven PH, Hong DY, editors. Flora of China. Vol. 23. Acoraceae through Cyperaceae. Beijing (China); St. Louis (MI): Science Press; Missouri Botanical Garden Press; p. 158–163.
- Su T, Yang JX, Lin YG, Kang N, Hu GX. 2019. Characterization of the complete chloroplast genome of Sparganium stoloniferum (Poales: Typhaceae) and phylogenetic analysis. Mitochondrial DNA Part B. 4(1):1402–1403.
- Zhang QY, Wang Y, Xu XW. 2021. The complete chloroplast genome of Sparganium fallax (Typhaceae). Mitochondrial DNA Part B. 6(7):2097–2098.