Abstract
Cyprinid fish Barilius barila found in the Irrawaddy water system is a valuable fishery resource and has been listed as Least Concern by the IUCN. This study determined the complete mitochondrial genome of B. barila from Yunnan, China, for the first time. Circular molecule of B. barila mitogenome was sequenced to be 16,560 bp in length, with the typical gene structure of 13 protein-coding genes, 22 transfer RNA genes, two ribosomal RNA genes, and two noncoding areas (control region and the origin of L-strand replication). Overall nucleotides composition appeared to be 27.5% A, 24.8% T, 19.2% G, and 28.6% C, with a slight AT (52.3%) bias. The topology of the phylogenetic tree showed that B. barila was well grouped with Opsarius caudiocellatus, and clustered together with the genus Opsarius instead of Barilius, revealing that it was more reasonable for Barilius barila to belong to Opsarius rather than Barilius.
1. Introduction
The cyprinid fish, Barilius barila (Hamilton, 1822) belongs to the Chedrinae subfamily of the Danionidae family and is endemic to the Irrawaddy water system with ornamental and economic value. It inhabits large hill streams and shallow clear rivers along foothills. It can be easily distinguished based on the morphological features as follows (): dorsal fin iii-7-8, anal fin ii-10-11, pectoral fin i-11-12, ventral fin i-7-8; 22 predorsal scales, 40–42 + 2–3 lateral line scales, pectoral fin as long as head, silvery white body with 11–15 blue patches on both sides (Chu Citation1984; Prabhu et al. Citation2020). Due to a great reduction in natural population number, B. barila has been assessed as Least Concern (LC) status in the IUCN Red List of Threatened Species in 2010. Recently, there were few Barilius species mitogenome sequences reported in public. The present study examined the complete mitochondrial sequence of B. barila and investigated the phylogenetic relationships within Chedrinae for the first time, which would be advantageous in DNA barcode development and targeted conservation.
2. Materials and methods
2.1. Sample collection and preservation
Experiments in this study complied with the recommendations of the Ethics Committee for Animal Experiments of Jiangsu Agri-animal Husbandry Vocational College. The B. barila sample was obtained from Da Ying River in Yingjiang County, Dehong Prefecture, Yunnan Province of China (24°69′05.95″ N, 97°94′89.69″E) on 8 July 2022. The obtained fish specimens were euthanized, first anesthetized using a concentration of 0.2 mL/L eugenol solution, then placed into 75% ethanol for fixation and finally transferred to 95% ethanol for long-term storage. All specimens were deposited in the fish collection at Aquatic Science and Technology Institution Herbarium (https://www.jsahvc.edu.cn/; Deposit number ASTIH-21b1108d28; Chen Xiao Jiang, [email protected]).
2.2. Mitochondrial genome sequencing and phylogenetic analysis
The muscle tissue was collected for genomic DNA isolation using the Tguide Cell/tissue genomic DNA Extraction Kit (OSR-M401) (Tiangen, Beijing, China). After DNA sample quality control, a DNA library was constructed and amplified by PCR, followed by size selection and library quality check, finally the amplified original library DNA was subjected to Illumina HiSeq 4000 Sequencing platform (Illumina, CA). The sequenced fragments were processed for the quality check to filtrate low-quality reads on FastQC Version 0.11.8 (Andrews Citation2015), and then assembled into a circular mitogenome of B. barila by MetaSPAdes 3.13.0 (Nurk et al. Citation2017) with Barilius malabaricus MN650735 as reference, and then the assembled mitochondrial genome sequences were annotated using MitoMaker 1.14 (Bernt et al. Citation2013) with default parameters. The resulting complete mitochondrial genome was deposited in GenBank with accession number OM617728. OGDRAW (https://chlorobox.mpimp-golm.mpg.de/OGDraw.html) was used to draw the genome maps (Greiner et al. Citation2019). Alignments, analysis, model calculation, and phylogenetic reconstruction were all completed by MEGA X (Kumar et al. Citation2018).
3. Results and discussion
3.1. Mitochondrial DNA genome structure
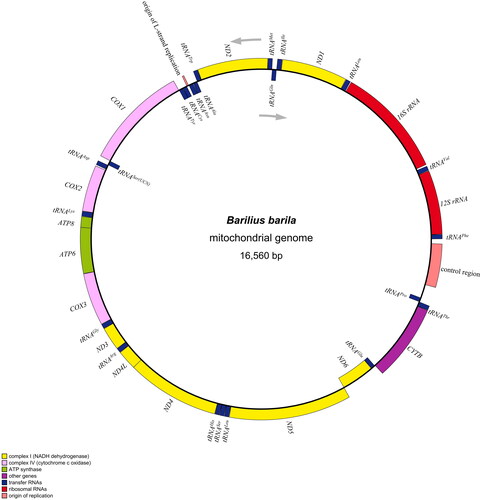
The B. barila mitogenome had a closed double-stranded circular molecule with 16,560 bp in length and essentially contained 13 protein-coding genes, 22 transfer RNA genes, two ribosomal RNA genes, and two noncoding areas (a control region and an origin of L-strand replication), resembling those of other Danionidae species (Chen et al. Citation2022; Song et al. Citation2022). The overall nucleotide contents of A, T, G, and C appeared to be 27.5%, 24.8%, 19.2%, and 28.6% respectively, thereby with a slight AT (52.3%) bias. Most mitochondrial genes were encoded on H-strand except for ND6 and eight tRNA genes (tRNAGln, tRNAAla, tRNAAsn, tRNACys, tRNATyr, tRNASer(UCN), tRNAGlu, and tRNAPro), which were encoded on the L- strand (). All PCGs initiated with normal ATG except for CO1 with GTG as its start codon. Besides, the stop codon usage patterns were diverse: most PCGs (ND1, CO1, ATP8, ATP6, CO3, ND3, ND4L, and ND5) terminated with routine TAA codon, and two genes (ND2 and ND6) utilized TAG as the stop codon, while the remaining three PCGs ended by incomplete TA (ND4) or single T (CO2 and Cytb), which was a common feature among vertebrate mitogenomes (Luo et al. Citation2019; Tan et al. Citation2020). Sixteen intergenic spacers were found in the whole mitogenome, ranging from 1 to 33 bp in length. Simultaneously, ten reading frame overlaps were observed with the largest overlap of 7 nucleotides in two sites (ATP6-ATP8 and ND4L-ND4). The size of 22 tRNA genes varied from 66 bp (tRNACys) to 75 bp (tRNALys), while the control region extended up to 382 nucleotides and was identified between tRNAPhe and tRNAPro. The subunits of rRNA in B. barila genome were of two types, namely 12S rRNA and 16S rRNA, with lengths of 954 bp and 1653 bp respectively.
Figure 2. Mitochondrial genome map of Barilius barila (Genbank no. OM617728), consisting of 13 PCGs, 22 tRNAs, two rRNAs, an origin of L-strand replication and a control region. The arrows represents direction of transcription. tRNAGln, tRNAAla, tRNAAsn, tRNACys, tRNATyr, tRNASer(UCN), tRNAGlu, tRNAPro, and ND6 encoded on L-strand are displayed inside the circle, the other genes encoded on H-strand are displayed outside the circle.
3.2. Phylogenetic analysis
Amino acid sequences of 13 protein-coding genes of B. barila were aligned on MEGA X with that of other 19 species of fish from 9 genera (Opsarius, Barilius, Raiamas, Opsaridium, Leptocypris, Salmostoma, Cabdio, Luciosoma, Rasbora) available in Genbank (Tang et al. Citation2010; Saitoh et al. Citation2011; Chang et al. Citation2013; Kusuma and Kumazawa Citation2016; Miya et al. Citation2016; Prabhu et al. Citation2020; Chen et al. Citation2022; Yu et al. Citation2022). Rasbora lateristriata and Rasbora steineri were selected as outgroups. The best evolutionary model was simulated to be GTR + G + I for it has obtained the lowest Bayesian information standard scores (Nei and Kumar Citation2000). The ML analysis generated topological structure and the phylogenetic position of B. barila in subfamily Chedrinae was shown in . All 18 Chedrinae fish species were split into three well-supported major clades, Clade A (A1 + A2 + A3) and Clade B and Clade C. Clade A2 (Raiamas guttatus, Barilius ardens, Barilius malabaricus, and Opsarius canarensis) became the sister-group with the Clade A3 (Opsaridium ubangiense, Raiamas buchholzi, Leptocypris sp, Raiamas senegalensis, and Raiamas steindachneri), and they formed a sister-group with the Clade A1 (Opsarius bendelisis, Opsarius pulchellus, Opsarius bernatziki, Barilius barila, and Opsarius caudiocellatus). Further, it showed that B. barila was clustered together with genus Opsarius, especially well grouped with Opsarius caudiocellatus.
Figure 3. Maximum-likelihood (ML) phylogenetic tree reconstructed using concatenated mitochondrial protein-coding genes of B. barila and other 19 fish species. Accession numbers were followed by the name of the species. The tree topology was evaluated by 1000 bootstrap replicates. Bootstrap values at the nodes correspond to the support values for ML methods.
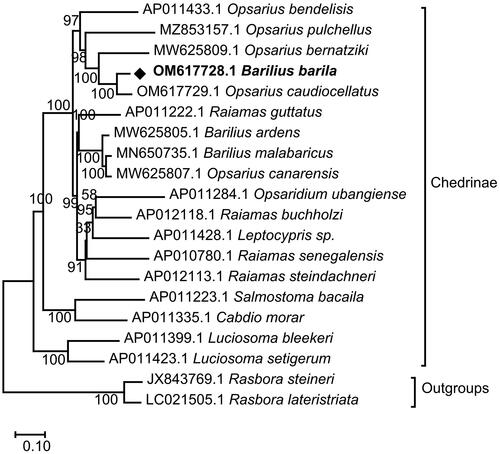
The classification of the genus Barilius was very complex. Barilius (sensu lato) was divided into the genera Barilius and Opsarius by Howes (Citation1980). Qin et al. have proposed that the genus Barilius from South Asia should be changed to Opsarius based on morphology and molecular phylogeny, such as Barilius canarensis was changed to Opsarius canarensis (Qin et al. Citation2019). The phylogenetic tree showed that it was more reasonable for Barilius barila to belong to the genus Opsarius. In addition, Opsarius canarensis was clustered together with the genus Barilius instead of Opsarius, which was incongruent with the viewpoint of Qin et al. It was recommended that more data be needed for further analysis and confirmation.
4. Conclusions
This study determined the complete mitochondrial genome of B. barila for the first time using the high-throughput sequencing technology. The assembly circular mitogenome was 16,560 bp long (deposited in GenBank with accession number OM617728). The phylogenetic tree was constructed based on the maximum likelihood method showed that B. barila was clustered together with the genus Opsarius, revealing that it may be more reasonable for B. barila to belong to the genus Opsarius instead of Barilius. This mitochondrial genome would establish a basis for furthering research on species evolution and phylogenetic of the subfamily Chedrinae, it would also be conducive to the development of the DNA barcode and targeted conservation.
Ethical approval
Experiments were performed in accordance with the recommendations of the Ethics Committee for Animal Experiments of Jiangsu Agri-animal Husbandry Vocational College. These policies were enacted according to the Chinese Association for the Laboratory Animal Sciences and the Institutional Animal Care and Use Committee (IACUC) protocols.
Author contributions
Xiao Jiang Chen and Lin Song make substantial contributions to the conception or design of the work, and drafting the paper, and Final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; Yang Song and Quan Wang were involved in the acquisition, analysis and interpretation of the data; the drafting of the paper, and the final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The contributions are ranked in order.
Supplemental Material
Download JPEG Image (485.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under the reference number OM617728. The associated “BioProject”, “Bio-Sample” and “SRA” numbers are PRJNA808197, SAMN26036042, and SRR18066595 respectively.
Additional information
Funding
References
- Andrews S. 2015. FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- Bernt M, Donath A, Juhling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Chang CH, Tsai CL, Jang-Liaw NH. 2013. Complete mitochondrial genome of the Chinese rasbora Rasbora steineri (Teleostei, Cyprinidae). Mitochondrial DNA. 24(3):183–185.
- Chen XJ, Song L, Liu WZ, Gao P. 2022. Characterization of the complete mitochondrial genome and phylogenetic analysis of Opsarius pulchellus (Cypriniformes, Danionidae, Chedrinae). Mitochondrial DNA B Resour. 7(4):666–668.
- Chu XL. 1984. Provisional revision of the genus Barilius in China (Pisces: Cyprinidae). Zoological Research. 5(1):95–102.
- Greiner S, Lehwark P, Bock R. 2019. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 47(W1):W59–W64.
- Howes GJ. 1980. The anatomy, phylogeny, and classification of bariliine cyprinid fishes. Bull Brit Museum Nat Hist Zool. 37:129–198.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol. 35(6):1547–1549.
- Kusuma WE, Kumazawa Y. 2016. Complete mitochondrial genome sequences of two Indonesian rasboras (Rasbora aprotaenia and Rasbora lateristriata). Mitochondrial DNA A DNA Mapp Seq Anal. 27(6):4222–4223.
- Luo F, Huang J, Luo T, Yang J, Zhou H, Wen Y. 2019. Complete mitochondrial genome and phylogenetic analysis of Yunnanilus pulcherrimus (Cypriniformes, Nemacheilidae). Mitochondrial DNA B. 4(1):1269–1270.
- Miya M, Minamoto T, Yamanaka H, Oka SI, Doi H. 2016. Use of a filter cartridge for filtration of water samples and extraction of environmental DNA. J Vis Exp Jove. (117):1–8.
- Nei M, Kumar S. 2000. Molecular evolution and phylogenetics. New York (NY): oxford University Press.
- Nurk S, Meleshko D, Korobeynikov A, Pevzner PA. 2017. MetaSPAdes: a new versatile metagenomic assembler. Genome Res. 27(5):824–834.
- Prabhu VR, Singha HS, Kumar RG, Gopalakrishnan A, Nagarajan M. 2020. Characterization of the complete mitochondrial genome of Barilius malabaricus and its phylogenetic implications. Genomics. 112(3):2154–2163.
- Qin T, Maung KW, Chen XY. 2019. Opsarius putaoensis, a new species of subfamily Danioninae (Actinopterygii, Cyprinidae) from the Irrawaddy River basin in northern Myanmar. Zootaxa. 4615(3):zootaxa.4615.3.11–593.
- Saitoh K, Sado T, Doosey MH, Bart HL Jr, Inoue JG, Nishida M, Mayden RL, Miya M. 2011. Evidence from mitochondrial genomics supports the lower Mesozoic of South Asia as the time and place of basal divergence of cypriniform fishes (Actinopterygii: Ostariophysi). Zool JLinn Soc. 161(3):633–662.
- Song L, Chen XJ, Han XP, Gu YW. 2022. Complete mitochondrial genome and phylogenetic analysis of Danio roseus (Teleostei, Cypriniformes, Danionidae). Mitochondrial DNA B Resour. 7(2):350–352.
- Tan HY, Yang YY, Zhang M, Chen XL. 2020. The complete mitochondrial genome of Rhinogobius duospilus (Gobiidae: Gobionellinae). Mitochondrial DNA B Resour. 5(3):3406–3407.
- Tang KL, Agnew MK, Hirt MV, Sado T, Schneider LM, Freyhof J, Sulaiman Z, Swartz E, Vidthayanon C, Miya M, et al. 2010. Systematics of the subfamily danioninae (Teleostei: Cypriniformes: Cyprinidae). Mol Phylogenet Evol. 57(1):189–214.
- Yu P, Zhou L, Yang X-L, Li L-L, Jin H, Li Z, Wang Z-W, Li X-Y, Zhang X-J, Wang Y, et al. 2022. Comparative mitogenomic analyses unveil conserved and variable mitogenomic features and phylogeny of Chedrinae fish. Zool Res. 43(1):30–32.

