Abstract
In this study, the complete mitochondrial genome of Niwaella nigrolinea (Cypriniformes: Cobitidae) from Zhejiang, China, was determined for the first time. We found that the sequenced length of the complete mitochondrial genome of N. nigrolinea was 16,565 bp. The genome contained 13 protein-coding genes, 22 transfer RNAs, two ribosomal RNAs, and two non-coding regions, identical to most other vertebrates. Our phylogenetic analysis results confirmed that N. nigrolinea was close to an unclassified Cobitis sp. and the fishes of the genus Cobitis. These data would contribute to the genetic conservation genetics and stock evaluation of N. nigrolinea.
Loaches of the genus Niwaella Nalbant, 1963 (Cypriniformes, Cobitidae) are small benthic freshwater fishes found throughout East Asia, including China, Korea, and Japan. The fish inhabits the pebble or boulder bottoms of clear, swift-moving mountain streams, lack sexual dimorphism, and have extremely elongated bodies (Chen et al. Citation2017). Niwaella nigrolinea (Chen et al. Citation2017) was discovered in Xin’an River, Anhui Province, China, in 2017. Chen et al. (Citation2017) named it N. nigrolinea. However, at present, N. nigrolinea and Cobitis nigrolinea are considered synonyms (Perdices et al. Citation2016). Compared to similar species, N. nigrolinea has a black stripe from the occiput to the caudal fin on the dorsum, short and dense vertical bars on the dorsolateral surface, a split lower lip and mandible, undeveloped mental lobes, a thick and curved suborbital spine, and a long processus latero-caudalis and caudal peduncle (Chen et al. Citation2017). In addition, Chen et al. (Citation2017) obtained 1140 bp of the mitochondrial cytochrome b gene and identified three unique haplotypes in three specimens of N. nigrolinea. However, to our knowledge, no publication exists on the mitochondrial genome of N. nigrolinea. Here, we report the complete mitochondrial genome of N. nigrolinea for the first time and the results of our evaluation of its phylogenetic performance among Cobitidae fishes.
Niwaella nigrolinea was collected from a stream beside Yunyuan Port in Chun’an County, Zhejiang Province, China (118°54′14.5′′ E, 29°49′41.5′′ N), and deposited at the National Original Breeding Farm of black Amur bream from China’s Qiantang River (120°07′21.99″E, 30°08′35.53″N). The phenol-chloroform extraction method (Green and Sambrook Citation2012) was applied to obtain total genomic DNA from the fin tissue deposited at Hangzhou Academy of Agricultural Sciences (http://www.hznky.com/, Kai Liu and [email protected]) under the voucher number HXHQ202009. After quantifying the genomic DNA, it was sonicated using a Covaris M220 Ultrasonicator™ (Covaris, Inc., Woburn, MA, USA). Purified sheared DNA fragments were used to build a sequencing library and then subjected to next-generation sequencing (NGS). The NGS was performed by Origingene Bio-pharm Technology Co., Ltd. (Shanghai, China). The mitochondrial genome of N. nigrolinea was obtained by sequence assembly of the NGS data using NOVOPlasty ver. 4.3.1 (Dierckxsens et al. Citation2017). The accession number MZ707538 was assigned to the genome by GenBank. The MITOFISH prediction server (Iwasaki et al. Citation2013) was used to complete the annotation process.
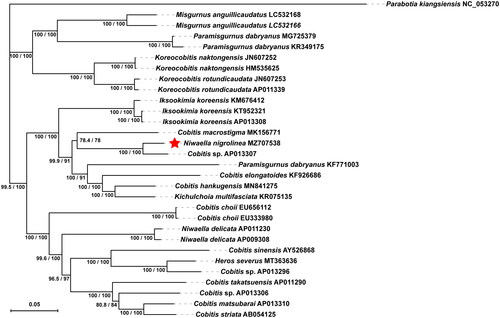
The complete mitochondrial genome of N. nigrolinea from Yunyuan Port was identified to have a length of 16,565 bp and to contain 22 transfer RNAs (tRNAs), 2 ribosomal RNAs (rRNAs), 13 protein-coding genes (PCGs), and 2 non-coding regions. It is identical to those of most vertebrate mitochondrial genomes. The base composition of the mitochondrial genome of N. nigrolinea was found to consist of 29.39% A, 27.98% T, 25.70% C, and 16.93% G, with an AT content of 57.37%. Most PCGs and RNAs of N. nigrolinea are encoded on the heavy strand (H-strand) except for ND6 and eight tRNAs encoded on the light strand (L-strand). Apart from CO1, which was initiated with GTG, all 13 PCGs inside the genome had the standard start codon ATG. However, the stop codons of the 13 PCGs varied, concluding with TAG, TAA, TA − or T−. The origin of light-strand replication (OL), which could span up to 31 nucleotides, was established in the WANCY region (containing trnW, trnA, trnN, trnC, and trnY). The second non-coding region, the control region (D-loop), had a length of 912 bp and was positioned between the tRNA trnP and trnF. , drawn by IQ-TREE ver. 2.1.3, depicts the phylogenetic tree of N. nigrolinea, using the 13 PCGs and the TPM2 + F + I + G4 substitution model (Minh et al. Citation2020). The Ultrafast Bootstrap method (repeated 1000 times) was used to test the reliability of the phylogenetic tree (Hoang et al. Citation2018). SH-aLRT values and Ultrafast Bootstrap values are expressed in percentages. The phylogenetic analysis showed that N. nigrolinea (MZ707538) initially clustered with an unclassified Cobitis sp. (AP013307) into a branch with a high bootstrap value. Then, they clustered with Cobitis macrostigma (MK156771) with a lower bootstrap value and with Cobitis hankugensis (MN841275) and Cobitis elongatoides (KF926686). However, N. nigrolinea (MZ707538), C. macrostigma (MK156771), and the other aforementioned fishes of the genus Cobitis were not first grouped with the other fishes of the genus Cobitis, including C. choii (EU656112), C. sinensis (AY526868), and C. striata (AB054125), but after the genus Iksookimia fishes. Fishes of other genera, such as Koreocobitis, Paramisgurnus, and Misgurnus, can be clustered in different branches from those of the genus Cobitis. It should be noted that Paramisgurnus dabryanus (KF771003) was not grouped with other P. dabryanus fish, and Kichulchoia multifasciata (KR075135) was placed in a group with fishes of the genus Cobitis. In addition, Heros severus (MT363636) representatives are in the same clade as some fishes of the genus Cobitis, which requires further scientific attention.
Authors’ contributions
Tian-Jiang Chu and Kai Liu carried out the experiments; Tian-Jiang Chu and Ning Yang assisted in sample collection; Tian-Jiang Chu assisted in sample sequencing; Kai Liu analyzed the data and wrote the manuscript. The final manuscript was reviewed and approved by all authors.
Ethical approval
Approval from the Science and Technology Bureau of China and the Department of Wildlife Administration is not required for the experiments conducted in this paper when the fish in question are neither rare nor near extinction (first- or second-class state protection level). According to Measures of Zhejiang Province on Administration of Laboratory Animals, ethical approval was not required because the approval is only necessary when researchers will use mammals.
Disclosure statement
The authors reported no potential conflict of interest.
Data availability statement
The data supporting this study’s findings are openly available in National Center for Biotechnology Information (NCBI) at https://www.ncbi.nlm.nih.gov/nuccore, reference number MZ707538. The associated BioProject, SRA, and BioSample numbers are PRJNA761196, SRR15734596, and SAMN21245545, respectively.
Additional information
Funding
References
- Chen Y, He D, Chen H, Chen Y. 2017. Taxonomic study of the genus Niwaella (Cypriniformes: Cobitidae) from East China, with description of four new species. Zool Syst. 42:490–507.
- Dierckxsens N, Mardulyn P, Smits G. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45(4):e18.
- Green MR, Sambrook J. 2012. Molecular cloning: a laboratory manual. 4th ed. New York (NY): Cold Spring Harbor Laboratory Press.
- Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the Ultrafast Bootstrap approximation. Mol Biol Evol. 35(2):518–522.
- Iwasaki W, Fukunaga T, Isagozawa R, Yamada K, Maeda Y, Satoh TP, Sado T, Mabuchi K, Takeshima H, Miya M, et al. 2013. MitoFish and MitoAnnotator: a mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol Biol Evol. 30(11):2531–2540.
- Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R. 2020. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 37(5):1530–1534.
- Perdices A, Bohlen J, Slechtova V, Doadrio I. 2016. Molecular evidence for multiple origins of the European spined loaches (Teleostei, Cobitidae). PLOS One. 11(1):e0144628.