Abstract
Ajuga spectabilis Nakai is a Korean endemic species in Lamiaceae. In spite of its importance, genomic studies are not performed on this species. Here, we report the complete plastid genome sequences of A. spectabilis, which will provide valuable information for its natural conservation and future studies for the plastid genome evolution. The plastid genome is 150,417 bp in length, containing a large single-copy region (LSC) of 82,140 bp and a small single-copy (SSC) region of 17,165 bp which are separated by a pair of inverted repeats (IR) of 25,556 bp. It encodes 113 genes, including 79 protein-coding genes, 30 tRNA genes, and four rRNA genes. The overall GC content is 38.3%, and those in the LSC, SSC, and IR regions are 36.4%, 32.2%, and 43.3%, respectively, which is consistent with other Ajuga species. Our phylogenetic analysis revealed that A. spectabilis formed a close relationship with A. ciliata and A. decumbens.
Introduction
Endemic species are crucial, as having unique genetic diversity for understanding evolution, biogeography, and speciation (Newmark and Newmark Citation2002; Cox et al. Citation2016). Various analyses have been performed to identify the plastid genome features and develop molecular markers to define the phylogenesis of endemic species (C. Kim et al. Citation2019; C. Kim et al. Citation2020). Lamiaceae Juss. are one of the largest family consisting of about 240 genera and 7000, species worldwide (Napoli et al. Citation2020) and is well-known for their therapeutic uses (Shinwari et al. Citation2013; Mamadalieva et al. Citation2017). Members of Lamiaceae were used in traditional medicine for circulatory, cutaneous, and musculoskeletal conditions (Zaman et al. Citation2022).
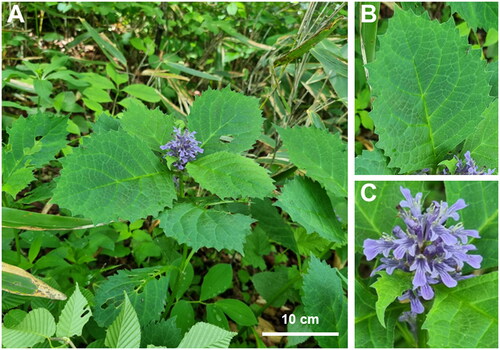
Ajuga is a significant genus with excellent medicinal and commercial qualities. A. spectabilis Nakai 1916 is an endemic perennial herb distributed in the mountains of South Korea except for Jeju Island (S.-Y. Kim et al. Citation2013). It is an upward-growing plant with broad ellipsoidal leaves (>8 cm in length) with deep violet flowers (Park Citation2007; S.-Y. Kim et al. Citation2013;) (). Medicinally, it stimulates smooth and cardiac muscle (Chung et al. Citation1980). Although it is an endemic species with known medicinal value, its genetic data have not been examined.
Figure 1. Photographs of A. spectabilis. (A). Perennial Plant; (B). ellipsoidal Leaves (10 cm); C. raceme inflorescence. The photographs were taken by Joonhyung Jung from Mt. Hwaya, Gapyeong-gun, Gyeonggi-do, South Korea, accessed on 20 May, 2021.
Chloroplast is a plant organelle that is crucial for photosynthesis and other biochemical processes (Cheng et al. Citation2020; Liang et al. Citation2020). Chloroplast genome (plastid genome) sequences are widely analyzed due to its highly conserved structure (Ravi et al. Citation2008). Plastid genome with a circular structure has four regions, large single copy (LSC), small single copy (SSC), and two inverted repeats (IR). Based on the plastid genome dataset, tribal classification was proposed for 12 subfamilies of Lamiaceae (Zhao et al. Citation2021). Here, we assembled the plastid genome sequences of A. spectabilis to provide valuable information for its natural conservation and phylogenetic relationship of Korean endemic plants.
Materials and methods
Plant materials and DNA extraction
We collected the individual of A. spectabilis from Mt. Hwaya, Gapyeong-gun, Gyeonggi-do, South Korea (N 37° 42′ 04.9″, E 127° 26′ 42.9″). The voucher specimen was deposited in the Gachon University herbarium (GCU) (Joonhyung Jung, email: [email protected]) under the accession number GCU210136644. Total genomic DNA was extracted using modified 2X cetyltrimethylammonium bromide (CTAB) method (Doyle and Doyle Citation1987). We measured DNA concentration using spectrophotometer (Biospec-nano; Shimadzu). The DNA purity was evaluated with 1.0% agarose gel (Table S2).
Genome sequencing, assembly and annotation
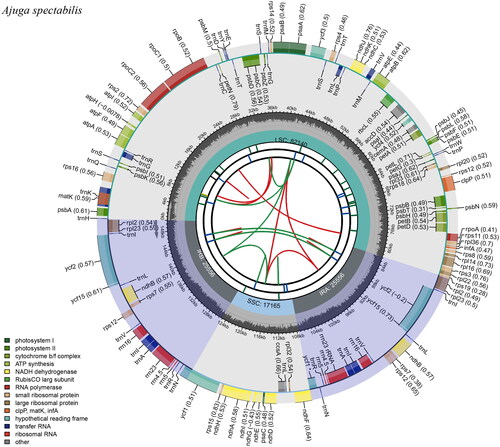
We performed the next-generation sequencing (NGS) using Illumina MiSeq Sequencer (Illumina, San Diego, California, USA). We imported the raw reads (10,781,982) and trimmed poor-quality reads using Geneious v.7.1.9 (Kearse et al. Citation2012). Then, we mapped to plastid genome of A. decumbens (GenBank accession No. MF967578). De novo assembly was implemented to generate consensus sequences and used as a reference for reassembling. We repeated this process until quadripartite structures were completed. Gaps were filled by Sanger sequencing using specific primers (Table S1). We annotated gene content and order using GeSeq (Tillich et al. Citation2017). All transfer RNAs were confirmed by tRNAScan-SE v.2.0 with the default search method (Lowe and Chan Citation2016). Complete plastid genome was illustrated using CPGview (http://www.1kmpg.cn/cpgview) ().
Figure 2. Complete plastid genome of A. spectabilis. The first circle shows the distributed repeats connected with red (transcribed clockwise) and green (transcribed counterclockwise) arcs from the center going outward. The second circle corresponds the tandem repeats marked with short bars. The next circle shows the microsatellite sequences as short bars. The fourth circle shows the size of the LSC and SSC. The fifth circle represents IRa and IRb. The sixth circle shows the GC contents within the plastid genome. The seventh circle defines the gene with different colors based on the functional group.
Phylogenetic analysis
We extracted a total of 79 protein-coding genes from 11 Ajugeae and two Clerodendreae species. Genes were aligned using MAFFT v.7 followed by manual modifications (Katoh et al. Citation2019). We performed maximum parsimony (MP), maximum likelihood (ML), and bayesian inference (BI) to reconstruct their phylogenetic relationships. Most parsimonious tree was searched with a heuristic algorithm in PAUP* v.4.0b10 (Swofford Citation2002). We used jModelTest v.2.1.7 (Guindon and Gascuel Citation2003; Darriba et al. Citation2012) to find the best model with Akaike’s information criterion (AIC) before running the ML and BI. The GTR + I + G was the best model for the concatenated dataset. ML analyses were implemented using the IQ-TREE web server (http://iqtree.cibiv.univie.ac.at/) (Nguyen et al. Citation2015). MrBayes v.3.2 was used for the BI analyses (Ronquist et al. Citation2012). MP bootstrap (PBP), ML bootstrap (MBP), and posterior probability (PP) were evaluated to estimate robustness for each clade. The phylogenetic trees were edited using FigTree v1.4.4 (Rambaut Citation2020).
Results
The complete plastid genome of A. spectabilis was 150,417 bp in length, consisting of the LSC (82,140 bp) and SSC (17,165 bp) separated by a pair of IRs (25,556 bp). It encodes 130 predicted functional genes, of which 113 were unique and 17 duplicated in the IR regions. The unique genes comprised 79 protein-coding genes, 30 tRNA genes, and four rRNA genes. The overall GC content was 38.3% and in the LSC, SSC, and IRs regions were 36.4%, 32.2%, and 43.3%, respectively.
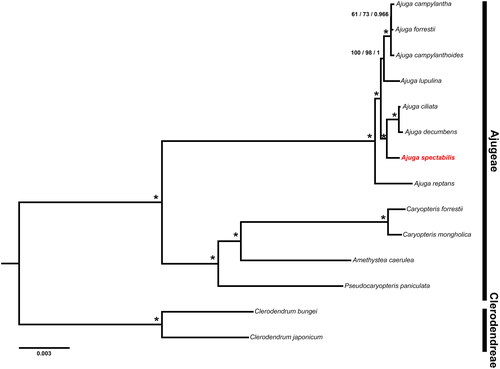
All MP, ML, and BI trees were identical in topology (). We identified the monophyly of Ajugeae with high support values (PBP and MBP = 100%, PP = 1.00). Also, A. spectabilis formed a close relationship with A. ciliata and A. decumbens.
Figure 3. Phylogenetic tree resulting from MP, ML, and BI based on 79 plastid protein-coding genes. A. spectabilis is highlighted in red and the accession no. is ON620078. Numbers indicate support (PBP/MBP/PP) and asterisks near nodes indicate PBP and MBP = 100% and PP = 1.00. The GenBank accession no. of sequences used in the study are Ajuga campylantha MN814851; Ajuga forresii MN518848 (Tao et al. Citation2019); Ajuga campylanthoides MN814852; Ajuga lupulina MN814856; Ajuga ciliata MN814853; Ajuga decumbens MF967578; Ajuga reptans KF709391 (Zhu et al. Citation2014); Caryopteris forrestii MT473742 (Zhao et al. Citation2021); Caryopteris mongholica NC035729 (Liu et al. Citation2018); Amethystea caerulea MN814858; Pseudocaryopteris paniculata MN814866; Clerodendrum bungei MW242824; Clerodendrum japonicum MT473745 (Zhao et al. Citation2021).
Discussion
Plastid genome sequences are widely used in phylogenetic implications, population genetic study, and species identification (Yang et al. Citation2013). Here, we assembled the complete plastid genome sequences of the Korean endemic, A. spectabilis. We identified that the genomic structures, gene contents and orders were highly conserved and similar to other Ajuga species. Our phylogenetic relationships revealed the monophyly of Ajugeae and were similar to the previous study with high support values (Zhao et al. Citation2021). Our results provide genetic resources for conservation and future evolutionary studies of Korean endemic species. Also, it may contribute to better resolving evolutionary relationships within phylogenetic clades of Lamiaceae.
Ethics approval and consent to participate
The material used in this study is widely distributed in Mt. Hwaya and does not belong to the IUCN Red List. No specific permits were required for plant collection. The study did not require ethical approval or consent, as no endangered or protected plant species were involved.
Credit authorship contribution statement
KK & JJ: Experimentation, Formal analysis, Data curation and interpretation, Writing- original draft. HJK & CYY: Project administration and design of the work. JHK: Conceptualization, Supervision, Writing-Review & Draft approval. All authors have read and approved the manuscript for publication, and all authors agreed to be accountable for all aspects of the work.
Supplemental Material
Download MS Excel (10.2 KB)Supplemental Material
Download MS Excel (16.6 KB)Acknowledgments
We would like to thank Tae-Hee Kim in Korean National Arboretum, and Ju Namgung in Gachon University (South Korea) for collecting samples used in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The complete chloroplast genome sequence we obtained from this study was archived in NCBI https://www.ncbi.nlm.nih.gov/nuccore/ON620078 under accession no. ON620078. The associated Bio project, SRA, and Bio-sample numbers are PRJNA847375, SRR19592548, and SAMN28933299 respectively.
Additional information
Funding
References
- Cheng Y, Zhang L, Qi J, Zhang L. 2020. Complete chloroplast genome sequence of Hibiscus cannabinus and comparative analysis of the Malvaceae family. Front Genet. 11:227.
- Chung B-S, Lee H-K, Kim J-W. 1980. Iridoid glycoside (I)-studies on the iridoid glycoside of Ajuga spectabilis Nakai. Korean J Pharmacogn.. 11(1):15–23.
- Cox CB, Moore PD, Ladle RJ. 2016. Biogeography: an ecological and evolutionary approach. London: John Wiley & Sons.
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 9(8):772–772.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15.
- Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol.. 52(5):696–704.
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 20(4):1160–1166.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649.
- Kim C, Do HDK, Jung J, Kim D-K, Kim J-H. 2020. Characterization of the complete chloroplast genome of Korean endemic, Habenaria cruciformis (Orchidaceae). Mitochondrial DNA Part B. 5(3):3269–3271.
- Kim C, Kim H-J, Do HDK, Jung J, Kim J-H. 2019. Characterization of the complete chloroplast genome of Fraxinus chiisanensis (Oleaceae), an endemic to Korea. Conservation Genet Resour. 11(1):63–66.
- Kim S-Y, Moon S-H, Kim J-S, Kim J-H, Lee BY. 2013. First record of Ajuga nipponensis Makino (Lamiaceae) from Korea. Korean J of Pl. 43(3):165–167.
- Liang H, Zhang Y, Deng J, Gao G, Ding C, Zhang L, Yang R. 2020. The complete chloroplast genome sequences of 14 Curcuma species: insights into genome evolution and phylogenetic relationships within Zingiberales. Front Genet. 11:802.
- Liu L, Zhang C, Wang Y, Dong M, Shang F, Li P. 2018. The complete chloroplast genome of Caryopteris mongholica and phylogenetic implications in Lamiaceae. Conserv Genet Resour. 10(3):281–285.
- Lowe TM, Chan PP. 2016. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 44(W1):W54–W57.
- Mamadalieva NZ, Akramov DK, Ovidi E, Tiezzi A, Nahar L, Azimova SS, Sarker SD. 2017. Aromatic medicinal plants of the Lamiaceae family from Uzbekistan: ethnopharmacology, essential oils composition, and biological activities. Medicines. 4(1):8.
- Napoli E, Siracusa L, Ruberto G. 2020. New tricks for old guys: recent developments in the chemistry, biochemistry, applications and exploitation of selected species from the Lamiaceae family. Chem Biodivers. 17(3):e1900677.
- Newmark WD, Newmark WD. 2002. Conserving biodiversity in East African forests: a study of the Eastern Arc Mountains (Vol. 155): Berlin: Springer Science & Business Media.
- Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274.
- Park C. 2007. The genera of vascular plants of Korea. Seoul: Academy publishing co.
- Rambaut A. 2020. FigTree v. 1.4. 4. 2018. Google Scholar.
- Ravi V, Khurana J, Tyagi A, Khurana P. 2008. An update on chloroplast genomes. Plant Systematics and Evolution. 271(1):101–122.
- Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542.
- Shinwari ZK, Ahmad N, Hussain J, Rehman NU. 2013. Antimicrobial evaluation and proximate profile of Nepeta leavigata, Nepeta kurramensis and Rhynchosia reniformis. Pak J Bot. 45(1):253–259.
- Swofford DL. 2002. PAUP: phylogenetic analysis using parsimony, version 4.0 b10. Sunderland, MA: Sinauer Associates.
- Tao A-E, Zhao F-Y, Xia C-L. 2019. Characterization of the complete chloroplast genome of Ajuga forrestii (Lamiaceae), a medicinal plant in southwest of China. Mitochondrial DNA Part B. 4(2):3969–3970.
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq – versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45(W1):W6–W11.
- Yang J-B, Tang M, Li H-T, Zhang Z-R, Li D-Z. 2013. Complete chloroplast genome of the genus Cymbidium: lights into the species identification, phylogenetic implications and population genetic analyses. BMC Evol Biol. 13(1):1–12.
- Zaman W, Ye J, Ahmad M, Saqib S, Shinwari ZK, Chen Z. 2022. Phylogenetic exploration of traditional Chinese medicinal plants: a case study on Lamiaceae (angiosperms). Pak J Bot. 54(3):1033–1040.
- Zhao F, Chen YP, Salmaki Y, Drew BT, Wilson TC, Scheen A-C, Celep F, Bräuchler C, Bendiksby M, Wang Q. 2021. An updated tribal classification of Lamiaceae based on plastome phylogenomics. BMC Biol. 19(1):2–27.
- Zhu A, Guo W, Jain K, Mower JP. 2014. Unprecedented heterogeneity in the synonymous substitution rate within a plant genome. Mol Biol Evol. 31(5):1228–1236.
