Abstract
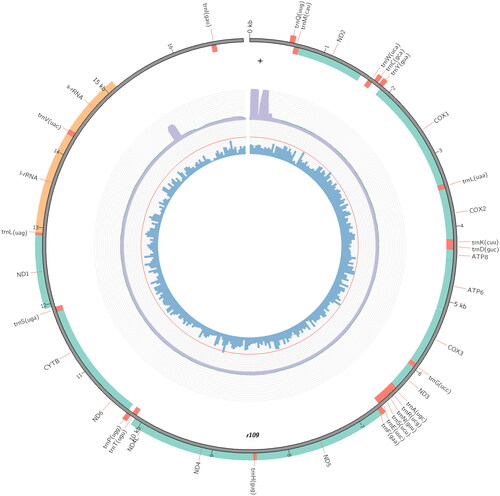
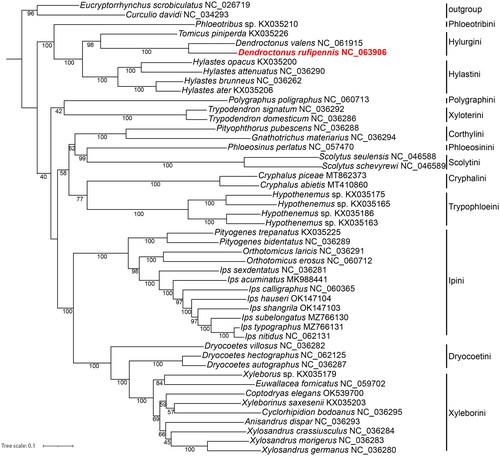
The mitogenome of Dendroctonus rufipennis is a circular molecule of 16,547 bp which consists of 13 protein-coding genes (PCGs), 22 transfer RNA genes, two ribosomal RNA genes, and a control region (GenBank accession no. NC_063906). All of 13 PCGs initiate with the standard start codon of ATN. Most PCGs used the typical stop codon ‘TAA’ or ‘TAG’, only nad5 terminated with incomplete stop codon (TA). Phylogenetic analyses within the Scolytinae were performed based on nucleotide sequences of mitochondrial PCGs. The topology showed that D. rufipennis, D. valens, and Tomicus piniperda formed a clade, and also clustered together with Hylastes opacus, H. attenuatus, H. brunneus, and H. ater. This indicated that there is a close genetic relationship between these species.
Dendroctonus rufipennis (Kirby, 1837) (Coleoptera: Curculionidae: Scolytinae) is one of the most destructive pests of spruce forests in North America. Under favorable conditions, outbreaks can develop and kill extensive forests over large areas (Werner et al. Citation1977). It is a quarantine pest in the list of entry plant quarantine pests of the People’s Republic of China and frequently intercepted in log quarantine at entry ports of China. To date, the mitogenome sequence of D. rufipennis remains unknown. Therefore, the mitochondrial genome of D. rufipennis was sequenced to provide more comprehensive data for this species.
The species is mainly identified by the following characteristics ( and ): adults are blackish-brown to black with reddish-brown or black elytra; range in length from 3.4 to 5.0 mm long and about 3 mm wide; frons without deep median groove between eyes, punctures usually obscure in central area; epistomal process narrower, distance between eyes about three times than its basal width, with moderately oblique lateral margins (anterior angles at 55°); elytra are 2.5 times the length of the pronotum; declivital striae at most weakly impressed, declivital striae 2 as wide or wider than 1 or 3, strial punctures on elytral declivity no more than two times as large as interstrial punctures (Wood Citation1982).
In this study, adult samples of D. rufipennis were intercepted in the Spruce logs import from British Columbia of Canada at Caofeidian Port (39°6′17″N, 118°30′29″E) in March 2020. The specimens were deposited at −20 °C in herbarium of Post-Entry Quarantine Station for Tropical Plant, Haikou Customs District P.R. China (specimen accession number: IN06130107-0001-0003, DNA voucher R109) (URL, Meng Rui, [email protected]). The mitogenome was sequenced using the Illumina HiSeq X TEN Sequencing System 2500 platform with 150 bp paired-end reads. The assembly method used here adopts the method of Meng et al. (Citation2019), and uses the Mitoz software package to conduct the assembly with default parameters. The annotations were mainly compared with the existing mitochondrial genomes of related species, and the annotation results were confirmed and modified by MITOS online tool (Bernt et al. Citation2013).
The nearly complete mitogenome sequence of D. rufipennis with a length of 16,937 bp (GenBank accession number NC_063906), contained 13 protein-coding genes (PCGs), 22 tRNA genes, two rRNA genes, and a control region (). There are 23 genes encoded on the heavy strand, while the remaining 14 genes encoded on the light strand. The nucleotide composition of the sequenced mitogenome sequence was biased toward AT 73.7%, and the total base composition was 40% A, 33.7% T, 10.6% G, and 15.6% C. The length of 13 PCGs was 11,132 bp, all PCGs started with ATN, ATA for atp8, cox3, nad3, cytb, and nad1; ATG for atp6, nad4, and nad4L; ATT for cox1, cox2, nad5, and nad6, ATC for nad2. Except for nad5 used TA as the stop codon, the remaining 12 PCGs were inferred to terminate with the complete stop codon (TAA or TAG). Twenty-two tRNA genes were identified and ranged in length from 61 bp to 70 bp. The large ribosomal gene (rrnL) is 1347 bp in length with an AT content of 80.2%, which was found between trnL1 and trnV. The small ribosomal gene (rrnS) is 809 bp with an AT content of 77.5%, and positioned between trnV and the control region. The major non-coding region between rrnS and trnI corresponding to control region, with A + T contents are 83%, which is obviously higher than the A + T content of the entire mitogenome. A conserved overlapping sequence motif ‘ATGATAG’ between ATP8 and ATP6 is present, this motif was also found in other mitogenomes from Scolytinae (Lv et al. Citation2017).
To validate the phylogenetic position of D. rufipennis based on mitogenome, we selected mitogenomes of 45 species which represent all Scolytinae 23 genera in 13 tribes currently deposited in the GenBank (). Two species respectively from Curculioninae and Cryptorhynchinae with close phylogenetic relationship to Scolytinae were selected as outgroup (Zhang et al. Citation2022). Nucleotide sequences were aligned, concatenated, and partitioned in Phylosuite (Katoh and Standley Citation2013; Zhang et al. Citation2020). Best partitioning scheme and evolutionary models for 13 pre-defined partitions were selected using PartitionFinder2 (Lanfear et al. Citation2017), with greedy algorithm and AIC criterion. The maximum-likelihood (ML) tree based on 13 PCGs was reconstructed in Phylosuite using partition model with 5000 bootstrap replicates (Nguyen et al. Citation2015; Zhang et al. Citation2020). The results showed that D. rufipennis, D. valens, and Tomicus piniperda in the Hylurgini formed a clade. The Hylurgini with Hylastini including Hylastes opacus, H. attenuatus, H. brunneus, and H. ater are clustered into one big branch. It indicated a close genetic relationship between these two tribes, this result was similar to the previous study (Johnson et al. Citation2018; Pistone et al. Citation2018). The independence of Trypophloeini is well supported in this study, in which tribal status has just been recovered in recent morphological study (Johnson et al. Citation2020). In conclusion, although this study provided important information for the phylogeny and evolution analysis in Scolytinae, more mt-genome sequences from all the 29 currently recognized tribes (Hulcr et al. Citation2015; Johnson et al. Citation2020) are also required in further studies to resolve the phylogenetic relationships within the whole subfamily.
Figure 4. Phylogenetic tree showing the relationship between D. rufipennis and other Scolytinae species based on ML methods. Numbers on branches are Bootstrap values. Eucryptorrhynchus scrobiculatus NC_026719 (Liu et al. Citation2016); Curculio davidi NC_034293 (Xu et al. Citation2017); Dendroctonus valens NC_061915 (Zhang et al. Citation2022); Ips calligraphus NC_060365 (Xu et al. Citation2021); Polygraphus poligraphus NC_060713, Orthotomicus erosus NC_060712, I. subelongatus MZ766130, I. typographus MZ766131, I. nitidus NC_062131, Dryocoetes hectographus NC_062125 (Du et al. Citation2021); I. hauseri OK147104, I. shangrila OK147103 (Du et al. Citation2022); Euwallacea fornicatus NC_059702 (Wang et al. Citation2020); Coptodryas elegans OK539700 (Guo et al. Citation2022); Phloeotribus sp. KX035210, Tomicus piniperda KX035226, Hylastes opacus KX035200, H. attenuatus NC_036290, H. brunneus NC_036262, H. ater KX035206, Trypodendron signatum NC_036292, T. domesticum NC_036286, Pityophthorus pubescens NC_036288, Gnathotrichus materiarius NC_036294, Phloeosinus perlatus NC_057470, Scolytus seulensis NC_046588, S. schevyrewi NC_046589, Cryphalus piceae MT862373, C. abietis MT410860, Hypothenemus sp. KX035175, KX035165, KX035186, KX035163, Pityogenes trepanatus KX035225, P. bidentatus NC_036289, Orthotomicus laricis NC_036291, I. sexdentatus NC_036281, I. acuminatus MK988441, Dryocoetes villosus NC036282, D. autographus NC_036287, Xyleborus sp. KX035179, Xyleborinus saxesenii KX035203, Cyclorhipidion bodoanus NC_036295, Anisandrus dispar NC_036293, Xylosandrus crassiusculus NC_036284, X. morigerus NC_036283, X. germanus NC_036280 (unpublished).
Author contributions
Rui Meng: the originator of the conception and design, original draft preparation. Hu Tian: the conception and design, analysis and interpretation of the data. Wei Lin: the conception and design, analysis and interpretation of the data. Yi-Xin Huang: the conception and design, analysis and interpretation of the data. Bo Cai: the conception and design, the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Ethics statement
The study involved only a common forest pest without any endangered or protect species. Therefore, no specific permits were required.
Disclosure statement
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Data availability statement
The data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/genbank/, reference number NC_063906. The associated BioProject, Bio-Sample numbers, and SRA are PRJNA874168, SAMN30527013, and SRR21245983, respectively.
Additional information
Funding
References
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Du HC, Fang JX, Shi X, Yu CM, Deng M, Zhang SF, Liu F, Zhang Z, Han FZ, Kong XB. 2022. Insights into the divergence of Chinese Ips bark beetles during evolutionary adaptation. Biology. 11(3):384.
- Du HC, Fang JX, Shi X, Zhang SF, Liu F, Yu CM, Zhang Z, Kong XB. 2021. Comparative analysis of eight mitogenomes of bark beetles and their phylogenetic implications. Insects. 12(10):949.
- Guo QH, Liu LY, Peng F, Sang W, Chen XS, Wang XM. 2022. First complete mitochondrial genome from the genus Coptodryas (Coleoptera: Curculionidae: Scolytinae) and its phylogenetic implications. Mitochondrial DNA B Resour. 7(4):575–576.
- Hulcr J, Atkinson TH, Cognato AI, Jordal BH, McKenna DD. 2015. Chapter 2 – morphology, taxonomy, and phylogenetics of bark beetles, pp 41–84. In: Vega FE, Hofstetter RW, editors. Bark beetles. Biology and ecology of native and invasive species. Cambridge (MA): Academic Press; 620 pp.
- Johnson AJ, Hulcr J, Knížek M, Atkinson TH, Mandelshtam MY, Smith SM, Cognato AI, Park S, Li Y, Jordal BH. 2020. Revision of the bark beetle genera within the former Cryphalini (Curculionidae: Scolytinae). Insect Syst Divers. 4(3):1–81.
- Johnson AJ, McKenna DD, Jordal BH, Cognato AI, Smith SM, Lemmon AR, Lemmon EM, Hulcr J. 2018. Phylogenomics clarifies repeated evolutionary origins of inbreeding and fungus farming in bark beetles (Curculionidae, Scolytinae). Mol Phylogenet Evol. 127:229–238.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. 2017. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol. 34(3):772–773.
- Liu ZK, Gao P, Ashraf MA, Wen JB. 2016. The complete mitochondrial genomes of two weevils, Eucryptorrhynchus chinensis and E. brandti: conserved genome arrangement in Curculionidae and deficiency of tRNA-Ile gene. Open Life Sci. 11(1):458–469.
- Lv F, Yang WY, Chen ZT, Xu Q, Zhou YJ, Du YZ. 2017. Three partial mitochondrial genomes from Ips (Coleoptera: Curculionidae, Scolytinae) contribute to the phylogeny of Scolytinae. J Asia-Pac Entomol. 20(3):1007–1013.
- Meng G, Li Y, Yang C, Liu S. 2019. MitoZ: a toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 47(11):e63.
- Nguyen L-T, Schmidt HA, Von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274.
- Pistone D, Gohli J, Jordal BH. 2018. Molecular phylogeny of bark and ambrosia beetles (Curculionidae: Scolytinae) based on 18 molecular markers. Syst Entomol. 43(2):387–406.
- Wang LJ, Hsu MH, Liu TY, Lin MY, Sung CH. 2020. Characterization of the complete mitochondrial genome of Euwallacea fornicatus (Eichhoff, 1868) (Coleoptera: Curculionidae: Scolytinae) and its phylogenetic implications. Mitochondrial DNA B Resour. 5(3):3520–3522.
- Werner RA, Baker BH, Rush PA. 1977. The spruce beetle in white spruce forests of Alaska. USDA Forest Service, Gen Tech Rep. PNW-61.
- Wood SL. 1982. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Gr Basin Nat Mem. 6:1359.
- Xu MF, Meng R, Shui KJ, Cai B, Lin W. 2021. The complete mitochondrial genome of Ips calligraphus (Germar 1824) (Coleoptera: Curculionidae: Scolytinae). Mitochondrial DNA B Resour. 6(9):2494–2495.
- Xu YD, Guan DL, Xu SQ. 2017. Characterization of the complete mitochondrial genome of the Chestnut weevil Curculio davidi (Insecta: Coleoptera: Curculionidae). Conserv Genet Resour. 9(2):1–4.
- Zhang D, Gao F, Li WX, Jakovlić I, Zou H, Zhang J, Wang GT. 2020. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour. 20(1):348–355.
- Zhang SN, Shi FM, Xu YB, Tao JX, Zong S. 2022. Characterization of the complete mitochondrial genome of Dendroctonus valens and elimination of nuclear mitochondrial pseudogene interference. J Appl Entomol. 146(7):801–812.