Abstract
Kappaphycus malesianus is a red seaweed farmed primarily for its carrageenan, a polysaccharide important in the food and pharmaceutical industries. Among the commercially cultivated Kappaphycus species, only K. malesianus has no mitogenome data available. Here, we assembled the mitochondrial genome of K. malesianus from next-generation sequencing data. The circular mitogenome consisted of 25,250 base pairs (bp) with a GC content of 30.25%. These values were comparable to previously sequenced solieriacean mitogenomes. Structural features, such as the stem-loop and hairpin, which were previously reported in other rhodophytes mitochondrial DNA, were also identified. The annotated genes (24 protein-coding genes, 24 tRNA genes, and 2 rRNA genes) were arranged in an order similar to the other available solieriacean mitogenomes. Lastly, phylogenetic analysis using 23 predicted protein domains showed the sister relationship of K. malesianus with other Kappaphycus species.
Introduction
Kappaphycus and Eucheuma are the most commercially important eucheumatoids. These two carrageenan-producing rhodophyte genera belong to the family Solieriaceae. They are the primary source of commercial carrageenan, a phycocolloid used in various food, pharmaceutical and material products (Guo et al. Citation2022). The food-grade carrageenan market is seen to reach a value of USD 1.2 billion by the end of 2022, and is projected to increase to USD 2.3 billion in the next decade (Future Market Insights Citation2022). Euchematoids are morphologically plastic, and their cultivars are often recognized by farmers based on visible traits such as habit, color, and branching pattern (Dumilag et al. Citation2023). The study by Roleda et al. (Citation2021) was unable to establish a link between genotype and morphology in Kappaphycus strains using the using cox1 and cox2-3 intergenic spacer sequences. Most cultivars were determined to belong to a single haplotype, which is likely due to the limited information provided by the short gene sequences (Lim et al. Citation2014; Dumilag et al. Citation2023). With the availability of genomic data, more information would be available, leading to the possibility of developing SNP markers beyond what is currently used. Among the commercially cultivated eucheumatoids, Kappaphycus malesianus J. Tan, P.E. Lim & S.M. Phang 2013 has been the least studied and has no genome data available. In this study, we present the assembled and annotated mitochondrial genome of K. malesianus.
Materials and methods
With the approval and supervision of the Local Government Unit (LGU) of Sitangkai, a specimen of K. malesianus was collected from Sapa-Sapa Bank, Sitangkai, Tawi-Tawi, Philippines (4°43'11.6’N 119°12'20.0’E). The voucher specimen () was identified as K. malesianus by R.V. Dumilag then deposited in the Mindanao State University Herbarium (MSU; [email protected]; http://sweetgum.nybg.org/science/ih/herbarium-details/?irn=259064) with the voucher number RD1484. DNA was extracted using a combination of modified CTAB (Zuccarello et al. Citation2006) and MagAttract HMW DNA kit (Qiagen) following the manufacturer’s protocols. The extracted DNA sample was sent to BGI Hong Kong for Library preparation using Illumina DNA PCR-Free Prep (Illumina, USA) and sequencing (150PE) using the Novaseq6000 platform (Illumina, USA). The complete mitogenomes of Kappaphycus striatus (NC024265) and Kappaphycus alvarezii (NC031814) were used as references during assembly and annotation. The genome was assembled using GetOrganelle (Jin et al. Citation2020) and the coverage depth was visualized using Bandage v0.8.1 (Wick et al. Citation2015). Annotations were done using AGORA (Jung et al. Citation2018) and tRNAscan-SE (Lowe and Chan Citation2016). Secondary structures were predicted using the RNAfold web server (Gruber et al. Citation2008). The final genome map was generated using OGDRAW (Greiner et al. Citation2019). To compare the gene order, Mauve 2.4.0 (Darling et al. Citation2004) was run using the progressive mode. For phylogenetic analysis, 23 mitochondrial protein sequences predicted from genes (atp4, atp6, atp8, atp9, cob, cox1, cox2, cox3, nad1, nad2, nad3, nad4, nad4L, nad5, nad6, rpl16, rps3, rps11, rps12, sdh2, sdh3, sdh4, and tatC) were aligned using Clustal Omega (Madeira et al. Citation2022) then concatenated. Gblocks 0.91b (Castresana Citation2000; Dereeper et al. Citation2008) was used to remove highly divergent regions of the alignment. The best-fit substitution model was selected using the IQ-TREE webserver (Trifinopoulos et al. Citation2016) and was determined to be MtMet + F + I + G (Le et al. Citation2017) using the Akaike information criterion (AIC). Phylogenetic trees were then inferred using maximum likelihood (ML) as implemented by IQ-TREE, and Bayesian inference (BI) using MrBayes 3.2.7 (Ronquist et al. Citation2012). The model used for BI was CpRev + I + F + G (Adachi et al. Citation2000) since MtMet was not available in MrBayes. In addition to the eucheumatoid sequences (Tablizo and Lluisma Citation2014; Li et al. Citation2018), sequences from the closely related species, Mastocarpus papillatus (Sissini et al. Citation2016), Riquetophycus sp. (Yang et al. Citation2015), and Chondrus crispus (Leblanc et al. Citation1995), were included in the phylogenetic tree construction.
Results
The K. malesianus mitogenome was assembled into a single circular structure of 25,250 bp with a depth of 144.7x (Figure S1) and a GC content of 30.25% (). Its size was longer than that of K. alvarezii and K. striatus (Tablizo and Lluisma Citation2014; Li et al. Citation2018) by 52 and 8 bp, respectively. The total number of genes was 50 (24 protein-coding genes, 24 tRNA genes and 2 rRNA genes), representing ∼94% of its total length (, S1 and S2). These genes were distributed almost evenly between the two mitochondrial DNA (mtDNA) strands, forming two transcriptional units in opposing directions (). At the junction of these units were two AT rich inverted repeats, which were also present in all the sequenced rhodophyte mitogenomes (e.g. Li et al. Citation2018). In the K. malesianus mitogenome, a 98-nucleotide stem-loop (ΔG = −67.70 kcal/mol) was found between the trnS2 and the trnA genes, while a 47-nucleotide hairpin (ΔG = −34.73 kcal/mol) was found between the cob and the trnL1 genes. The predicted protein products and their order appeared to be conserved within congeneric species (Figure S2). Similar to the other eucheumatoids, only 19 types of tRNAs were identified since the tRNA for threonine (Thr) was missing (Tablizo and Lluisma Citation2014; Li et al. Citation2018). The features of K. malesianus mitogenome as compared with those of other eucheumatoids are presented in .
Figure 2. Map of K. malesianus mitochondrial genome. Annotations outside the circle are in forward orientation, while those inside the circle are in reverse orientation. The GC content graph is illustrated inside the circle in gray.
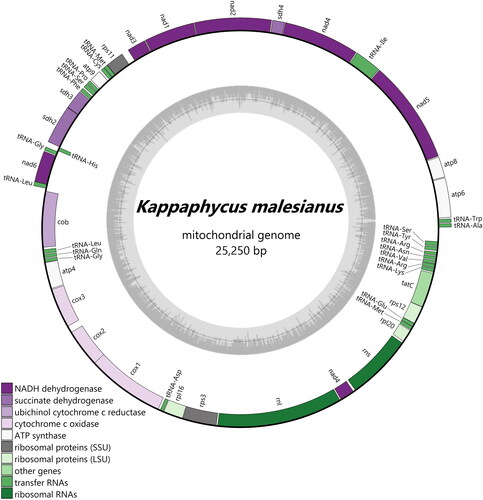
Table 1. General features of the mitochondrial genomes of five eucheumatoid species.
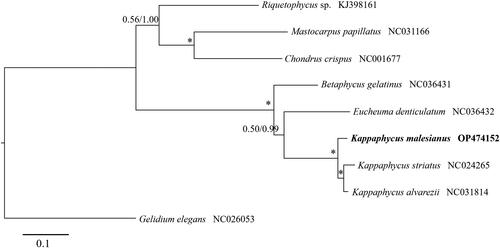
Phylogenetic analysis shows that K. malesianus is related to K. alvarezii and K. striatus (). Similar to previous reports, which used barcoding markers (Lim et al. Citation2014), our findings indicated stronger support of the sister relationship of K. alvarezii and K. striatus than that of K. malesianus with either taxon.
Figure 3. Maximum likelihood tree of nine rhodophytes based on 23 predicted mitochondrial proteins. The numbers above each node represent support values calculated from 1000 maximum likelihood bootstraps (left) and Bayesian posterior probability (right). Asterisk indicates a value of 1.00 for both supports. The scale bar indicates the number of substitutions per site.
Discussion and conclusion
The structure and organization of all the available Kappaphycus mitogenomes were conserved. The phylogenetic relationships of the farmed eucheumatoids inferred from mitochondrial proteins were consistent with previous DNA barcoding studies. Currently, the Indo-Pacific eucheumatoids are divided into five genera: Eucheuma, Betaphycus, Mimica, Kappaphycopsis, and Kappaphycus (Dumilag and Zuccarello Citation2022), with still unresolved intergeneric relationships. The inclusion of mitogenomes of other eucheumatoid taxa in future phylogenetic studies may resolve intergeneric relationships within the eucheumatoids.
Authors’ contributions
AOL, MYR, and RVD conceptualized the project. AOL and BAC designed the experiments. BAC performed the experiments and analyzed the data. BAC wrote the initial draft. AOL, MYR, and RVD revised the manuscript. All authors approved for this manuscript to be published and agreed to be accountable for all aspects of the work.
Supplemental Material
Download Zip (207.5 KB)Acknowledgements
The authors are grateful to Z.-Z. Aguinaldo, S. Damsik, and J. Turong for aiding during laboratory and field works. The authors also acknowledge the LGU of Sitangkai, Tawi-Tawi for granting permission for the collection activities. This is contribution no. 495 from the University of the Philippines the Marine Science Institute (UPMSI), Diliman, Quezon City.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The mitogenome sequence data that supported the findings in this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under accession no. OP474152. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA899665, SRR22244159, and SAMN31665931, respectively.
Additional information
Funding
References
- Adachi J, Waddell PJ, Martin W, Hasegawa M. 2000. Plastid genome phylogeny and a model of amino acid substitution for proteins encoded by chloroplast DNA. J Mol Evol. 50(4):348–358.
- Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 17(4):540–552.
- Darling ACE, Mau B, Blattner FR, Perna NT. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 14(7):1394–1403.
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard J-F, Guindon S, Lefort V, Lescot M, et al. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36(Web Server issue):W465–W469.
- Dumilag RV, Crisostomo BA, Aguinaldo Z-ZA, Hinaloc LAR, Liao LM, Roa-Quiaoit HA, Dangan-Galon F, Zuccarello GC, Guillemin M-L, Brodie J, et al. 2023. The diversity of eucheumatoid seaweed cultivars in the Philippines. Rev Fishs Sci Aquac. 31(1):47–65.
- Dumilag RV, Zuccarello GC. 2022. Phylogeny and genetic diversity of the Philippine eucheumatoid genus Mimica (Solieriaceae, Rhodophyta), and the proposal for Kappaphycopsis gen. nov. to include the anomalous species of Kappaphycus, K. cottonii. Phycologia. 61(5):496–503.
- Future Market Insights 2022. Food Grade Carrageenan Market [Internet]. [accessed 2022 Sep 24]. https://www.futuremarketinsights.com/reports/food-grade-carrageenan-market.
- Greiner S, Lehwark P, Bock R. 2019. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 47(W1):W59–W64.
- Gruber AR, Lorenz R, Bernhart SH, Neubock R, Hofacker IL. 2008. The Vienna RNA websuite. Nucleic Acids Res. 36(Web Server issue):W70–W74.
- Guo Z, Wei Y, Zhang Y, Xu Y, Zheng L, Zhu B, Yao Z. 2022. Carrageenan oligosaccharides: a comprehensive review of preparation, isolation, purification, structure, biological activities and applications. Algal Res. 61:102593.
- Jin J-J, Yu W-B, Yang J-B, Song Y, dePamphilis CW, Yi T-S, Li D-Z. 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21(1):241.
- Jung J, Kim JI, Jeong Y-S, Yi G. 2018. AGORA: organellar genome annotation from the amino acid and nucleotide references. Bioinformatics. 34(15):2661–2663.
- Le VS, Dang CC, Le QS. 2017. Improved mitochondrial amino acid substitution models for metazoan evolutionary studies. BMC Evol Biol. 17(1):136.
- Leblanc C, Boyen C, Richard O, Bonnard G, Grienenberger J-M, Kloareg B. 1995. Complete sequence of the mitochondrial DNA of the Rhodophyte Chondrus crispus(Gigartinales). Gene content and Genome organization. J Mol Biol. 250(4):484–495.
- Li Y, Liu N, Wang X, Tang X, Zhang L, Meinita MDN, Wang G, Yin H, Jin Y, Wang H, et al. 2018. Comparative genomics and systematics of Betaphycus, Eucheuma, and Kappaphycus (Solieriaceae: Rhodophyta) based on mitochondrial genome. J Appl Phycol. 30(6):3435–3443.
- Lim PE, Tan J, Phang SM, Nikmatullah A, Hong DD, Sunarpi H, Hurtado AQ. 2014. Genetic diversity of Kappaphycus Doty and Eucheuma J. Agardh (Solieriaceae, Rhodophyta) in Southeast Asia. J Appl Phycol. 26(2):1253–1272.
- Lowe TM, Chan PP. 2016. tRNAscan-SE on-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 44(W1):W54–W57.
- Madeira F, Pearce M, Tivey ARN, Basutkar P, Lee J, Edbali O, Madhusoodanan N, Kolesnikov A, Lopez R. 2022. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 50(W1):W276–W279.
- Roleda MY, Aguinaldo Z-ZA, Crisostomo BA, Hinaloc LAR, Projimo VZ, Dumilag RV, Lluisma AO. 2021. Discovery of novel haplotypes from wild populations of Kappaphycus (Gigartinales, Rhodophyta) in the Philippines. ALGAE. 36(1):1–12.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542.
- Sissini MN, Navarrete-Fernández TM, Murray EMC, Freese JM, Gentilhomme AS, Huber SR, Mumford TF, Hughey JR. 2016. Mitochondrial and plastid genome analysis of the heteromorphic red alga Mastocarpus papillatus (C. Agardh) Kützing (Phyllophoraceae, Rhodophyta) reveals two characteristic florideophyte organellar genomes. Mitochondrial DNA Part B Resour. 1(1):676–677.
- Tablizo FA, Lluisma AO. 2014. The mitochondrial genome of the red alga Kappaphycus striatus (‘Green Sacol’ variety): Complete nucleotide sequence, genome structure and organization, and comparative analysis. Mar Geonomics. 18:155–161.
- Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 44(W1):W232–W235.
- Wick RR, Schultz MB, Zobel J, Holt KE. 2015. Bandage: interactive visualisation of de novo genome assemblies. Bioinformatics. 31(20):3350–3352.
- Yang EC, Kim KM, Kim SY, Lee J, Boo GH, Lee J-H, Nelson WA, Yi G, Schmidt WE, Fredericq S, et al. 2015. Highly conserved mitochondrial genomes among multicellular red algae of the Florideophyceae. Genome Biol Evol. 7(8):2394–2406.
- Zuccarello GC, Critchley AT, Smith J, Sieber V, Lhonneur GB, West JA. 2006. Systematics and genetic variation in commercial shape Kappaphycus and shape Eucheuma (Solieriaceae, Rhodophyta). J Appl Phycol. 18(3-5):643–651.