Abstract
The complete mitochondrial genome of Drechslerella dactyloides was characterized in this study. This mitogenome is a closed circular molecule of 246860 bp in length with a GC content of 26.16%, including 87 predicted protein-coding genes, 29 transfer RNA genes, and two rRNA gens. Phylogenetic analyses based on concatenated amino acid sequences at 14 conserved mitochondrial protein-coding genes showed that D. dactyloides was closely related to Dactylellina haptotyla.
1. Introduction
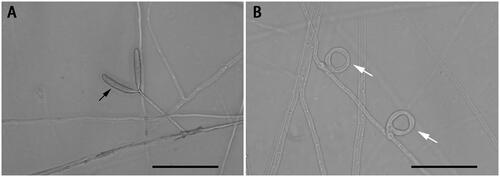
The nematode-trapping fungi are potential agents for the biological control of plant-parasitic nematodes (Morton et al. Citation2004). Their hypha can specialize into sophisticated structures called traps, including constricting rings, three-dimensional networks, adhesive hypha etc., which can catch nematodes (Hsueh et al. Citation2013). Drechslerella dactyloides (Drechsler) is a special nematode-trapping fungus that can capture nematodes using constricting rings (). A constricting ring is composed of three ring cells, with the aperture diameter at about 20 μm. When a nematode enters the ring and contacts the inner surfaces of the ring cells, the three ring cells rapidly triple their volume within 0.1 s and tighten the ring (Liu et al. Citation2012). The mechanism for ring cell inflation in such a short time and the genes involved in the formation and expansion of constricting ring cells are still poorly understood (Fan et al. Citation2021). Recently, the mitochondrial genomes have been reported from a number of nematophagous fungal species, which provided novel insights into the evolution of nematophagous fungi and facilitated further investigations of this ecologically and agriculturally important group of fungi (Zhang et al. Citation2020, Liang et al. Citation2021). In this study, we report the complete mitochondrial genome of D. dactyloides YMF1.00031 and investigate its phylogenetic relationship with other ascomycetous species, such as Fusarium spp., Aspergillus spp., Candida spp., etc.
2. Materials and methods
A specimen was collected from farmland (25°24′ N, 99°38′ E) and deposited at Microbial Library of the Germplasm Bank of wild species from Southwest China (http://www.genobank.org/, contact person: Ying Huang, [email protected]) under the voucher number YMF1.00031.
The mitogenome was part of our previously assembled whole genome of D. dactyloides. The sequencing was performed on Novaseq6000 and PacBio platform and assemblied by SOAPdenovo v. 2.0.4 and Canu v. 1.7 (unpublished data). All assembled contigs were compared to the local nt library through the local BLAST program, the threshold is set to e value <1e-5. Then manually check whether this contig has an overlap region in the first place and the end (to determine whether it is a circular sequence). Finally, the sequence was confirmed by nucleotide BLAST against the NCBI nr database.
The whole mitochondrial genome was annotated automatically using the Mitos2 tool (http://mitos2.bioinf.uni-leipzig.de/index.py) based on the Reference Ref 63 Fungi and Yeast 3 code (Bernt et al. Citation2013) and partially by manual annotation. tRNAs were annotated using tRNAscan-SE (Schattner et al. Citation2005). RepeatModeler and RepeatMasker were used for repeat sequence detection.
3. Results and discussion
The complete mitogenome of D. dactyloides is a closed circular molecule of 246860 bp. The reads’ coverage depth are shown in the Supplementary Figure S1. The GC content is 26.16%. It contains 151 predicted genes, including 87 mitochondrial protein-coding genes (PCGs), 29 tRNA genes, and two rRNA genes. Protein-encoding genes include three ATP synthase subunits (atp6, atp8, and atp9), three cytochrome oxidase subunits (cox1, cox2, and cox3), one apocytochrome b (cob), seven NADH dehydrogenase subunits (nad1 ∼ 4, nad4L, nad5 ∼ 6), one ribosomal protein (rps3) and 72 hypothetical proteins. All the protein coding genes and tRNA genes are encoded on the sense strand (). The total length of intergenic spacers is 97235 bp, acounts for 39% of the whole genome. A total of 47169 bp (19.11%) repeats were identified, of which 39747 bp were interspersed repeats and 7412 bp were tandem repeats. The presence of long intergenic regions and repeats may be the reason why the mitogenome is so large.
Figure 2. A circle diagram of the assembled mitochondria genome of Drechslerella dactyloides. [1] GC skew. green, GC skew -; red, GC skew+. [2] GC content. red, greater than the genome average GC content; green, less than the genome average GC content. [3] Protein coding genes (red), tRNAs (green) and rRNAs (blue).
![Figure 2. A circle diagram of the assembled mitochondria genome of Drechslerella dactyloides. [1] GC skew. green, GC skew -; red, GC skew+. [2] GC content. red, greater than the genome average GC content; green, less than the genome average GC content. [3] Protein coding genes (red), tRNAs (green) and rRNAs (blue).](/cms/asset/dd0b626d-3e6b-4954-81f1-6afb151cd547/tmdn_a_2197084_f0002_c.jpg)
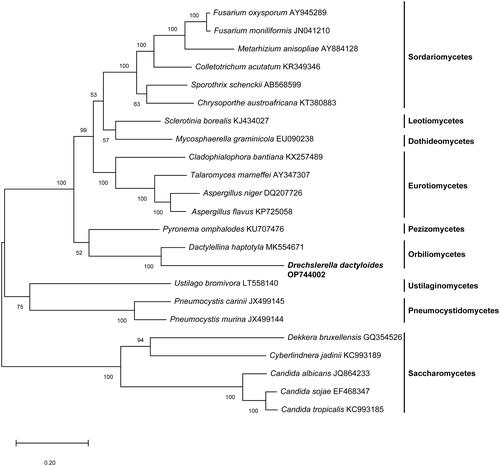
A maximum-likelihood tree of based on concatenated sequences of 14 conserved mitochondrial proteins of D. dactyloides and other 22 species from the Ascomycota was constructed by Mega-X (Kumar et al. Citation2018). As shown in , D. dactyloides was most closely clustered with Dactylellina haptotyla, a nematode-trapping fungus, which traps nematodes with sticky knobs and non-constricting loops ().
Figure 3. A maximum-likelihood tree of 23 fungal species based on concatenated amino acid sequences of 14 mitochondrial protein amino acid sequences. The 14 mitochondrial protein were: atp6, atp8, atp9, cox1, cox2, cox3, cob, nad1, nad2, nad3, nad4, nad4L, nad5 and nad6. The tree was generated using Mega-X and the best model was “WAG with Freqs. (+F).” Numerical values along branches represent bootstraps based on 1000 randomizations.
Ethical approval
This research does not involve ethical research. The fungus is not pathogenic and there are no ethics involved in using them in experiments.
Author contributions
L-ML and Y-RC were involved in conception and design; LZ collected and analyzed the data and drafted of the paper; L-ML analyzed the data; M-HM analyzed the data and revised the manuscript critically for important intellectual content and approved the final draft. All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download TIFF Image (2.7 MB)Supplemental Material
Download MS Word (572 KB)Acknowledgments
We would like to thank Prof. Jianping Xu at McMaster University, Canada, for his carefully language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
The authors declared no potential conflicts of interest with respect to the research, author- ship, and publication of this article.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at [https://www.ncbi.nlm.nih.gov] (https://www.ncbi.nlm.nih.gov/) under accession no. OP744002. The associated BioProject accession number is PRJNA916072; the Bio-Sample number is SAMN32411356; the SRA accession numbers are SRR23292935 and SRR23292936.
Additional information
Funding
References
- Bernt M, Donath A, Juhling F, Externbrink F, Florentz C, Fritzsch G, Putz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Fan Y, Zhang W, Chen Y, Xiang M, Liu X. 2021. DdaSTE12 is involved in trap formation, ring inflation, conidiation, and vegetative growth in the nematode-trapping fungus Drechslerella dactyloides. Appl Microbiol Biotechnol. 105(19):7379–7393.
- Hsueh Y-P, Mahanti P, Schroeder FC, Sternberg PW. 2013. Nematode-trapping fungi eavesdrop on nematode pheromones. Curr Biol. 23(1):83–86.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35(6):1547–1549.
- Liang LM, Zhang Y, Xu J, Zhang KQ, Cao YR. 2021. Characterization of the complete mitochondrial genome of the nematophagous fungus Purpureocillium lavendulum. Mitochondrial DNA Part B Resour. 6(1):33–35.
- Liu K, Tian J, Xiang M, Liu X. 2012. How carnivorous fungi use three-celled constricting rings to trap nematodes. Protein Cell. 3(5):325–328.
- Morton C, Hirsch P, Kerry B. 2004. Infection of plant-parasitic nematodes by nematophagous fungi - a review of the application of molecular biology to understand infection processes and to improve biological control. Nematol. 6(2):161–170.
- Schattner P, Brooks AN, Lowe TM. 2005. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33(Web Server):W686–W689.
- Zhang Y, Yang G, Fang M, Deng C, Zhang KQ, Yu Z, Xu J. 2020. Comparative analyses of mitochondrial genomes provide evolutionary insights into nematode-trapping fungi. Front Microbiol. 11:617.