Abstract
Paracondylactis sinensis Carlgren, 1934 (Actiniidae, Actiniaria) is an edible sea anemone in China. Their wild population has intensively decreased in recent years due to overharvesting. In this study, the complete mitochondrial genome of this economic species collected in the coast of Zhejiang, China is sequenced and obtained using high throughput methods. The total length of this circular molecule is 20,786 bp. Thirteen protein coding genes, two ribosomal RNA genes, two transfer RNA (tRNATrp, tRNAMet) genes and a putative ORF are annotated in it. Phylogenetic analysis based on the amino acids of mitochondrial genomes indicates that this species belongs to the family of Actiniidae. This result is consistent with the previous work that identified the edible sea anemone as Paracondylactis sinensis although it has always been recognized as Calliactis sinensis (of family Hormathiidae) in most Chinese reports. Overall, the mitochondrial genome produced in this study assists in clarifying the phylogenetic status of this sea anemone and provides a molecular foundation for future protection and breeding work.
1. Introduction
Paracondylactis sinensis Carlgren, Citation1934 (Actiniidae, Actiniaria) is a sea anemone distributed in the Indo-West Pacific Ocean (). In the coastal cities of southern China, particularly in Taizhou and Wenzhou cities of Zhejiang Province, P. sinensis has gained popularity as a delectable delicacy among the local residents. Unfortunately, the wild populations of P. sinensis have suffered significant declines due to overfishing and other human activities in recent years. As a result, the market price of P. sinensis has soared to 25 USD per kilogram (i.e. 170 CNY), making it an expensive cost for the locals to savor the dishes made of P. sinensis. Another factor to deteriorate the phenomenon is that the species identity of P. sinensis has always been mistaken for Calliactis sinensis (Verrill 1869) in many Chinese studies (Wu et al. Citation2011a–d), leading to the protection and maintaining of their wild population more difficult in China. Although taxonomists have revised the identity of this species (Pei Citation1998; Li Citation2013; Li and Xu Citation2020), the molecular information of P. sinensis is still lacked. In this study, specimens of P. sinensis were collected from the coasts of Taizhou and Wenzhou and the complete mitochondrial genome of P. sinensis was sequenced. Phylogenetic analysis based on mitochondrial genomes was performed to study the relationship between P. sinensis and other sea anemones. The result of this study will lay foundations for future studies on the population genetics, biogeography and genetic resource conservation of this edible sea anemone.
2. Materials and methods
Four individuals of P. sinensis were bought from seafood markets, two of which were sampled at the coast of Taizhou (121°39.5′E, 28°20.1′N) and the other two were collected at Nanji Islands of Wenzhou (121°04.2′E, 27°27.9′N). After preserved in pure alcohol for morphological observation and DNA extraction, all the samples were deposited in specimen room in the laboratory of marine organism taxonomy and phylogeny of the Institute of Oceanology, Chinese Academy of Sciences (http://www.qdio.ac.cn/motp/; Yang Li, [email protected]), under accession no. ss-Taizhou-1,ss-Taizhou-2, ss-Nanji-1, and ss-Nanji-2.
DNA was extracted from the body wall of the sample labeled as ss-Taizhou-1 using an E.Z.N.A.® Tissue DNA Kit (OMEGA, Wuhan, China) according to the manufacturer’s instructions. The procedures of high-throughput sequencing, sequence assembly, and gene annotation followed our previous works (Li et al. Citation2019a, Citation2019b). Briefly, a paired-end library with an insert size of 300 bp was prepared with total genomic DNA using the TruSeq DNA Sample Prep Kit (Illumina, USA). The above library was sequenced by an Illumina HiSeq Xten system (2 × 150 bp paired-end reads) (Illumina, USA) at Novogene Bioinformatics Technology Co., Ltd. (TianJin, China). Adapters and parts with a quality score below 15 were removed from raw reads by Trimmomatic 0.36 (Bolger et al. Citation2014). The clean reads were assembled using SPAdes 3.15.4 assembler (Bankevich et al. Citation2012) with default parameters. The mitochondrial genome was preliminarily annotated by the MITOS webserver (Bernt et al. Citation2013) and manually corrected. The read coverage depth of the produced mitochondrial genome was calculated with the Draw_SequencingDepth.py script provided by Ni et al. (Citation2023).
The amino acids of thirteen protein coding genes (PCGs) of P. sinensis and other 27 sea anemones (Huang Citation2009; Emblem et al. Citation2014; Foox et al. Citation2016; Stewart et al. Citation2017; Zhang and Zhu Citation2017; Chi et al. Citation2018; Surm et al. Citation2019; Wilding and Weedall Citation2019; Cong et al. Citation2020; Feng et al. Citation2021; Johansen et al. Citation2021) were concatenated for phylogenetic analysis. Among the 27 sea anemones, the PCGs of Calliactis polypus was obtained from its transcriptomic data (Stewart et al. Citation2017) and the PCGs of the other 26 sea anemones were extracted from their mitochondrial genomes. The phylogenetic tree was constructed using the PhyloSuite1.2.2 pipeline (Zhang et al. Citation2020). The maximum-likelihood phylogeny was inferred using IQ-TREE 1.6.8. with 20,000 ultrafast bootstraps (Nguyen et al. Citation2015). FLU + F + I + G4 model was selected as recommended by the built-in ModelFinder module in IQ-tree (Chernomor et al. Citation2016).
3. Results and discussion
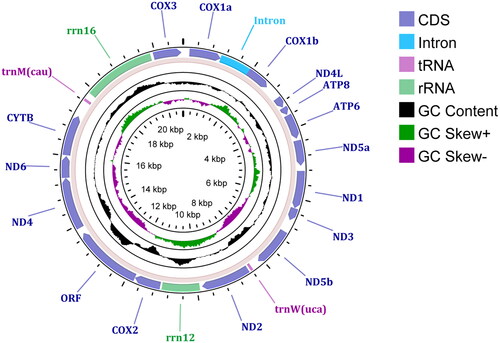
The assembled mitochondrial genome of P. sinensis is a circular molecule consisting of 20,786 bp (NCBI accession no. OP903146) with average sequencing depth of 140.71 × (Supporting Information Figure S1). Thirteen metazoan canonical PCGs engaged in energy production, a putative ORF, two ribosomal RNA genes and two transfer RNA genes (tRNA-Trp and tRNA-Met) are included in the mitochondrial genome. Introns can be found within the genes of cox1 and nad5 (). The components, structures, and arrangements of these genes are consistent with other mitochondrial genomes within the subclass Hexacorallia (Li et al. 2019).
Figure 2. A circular genomic map of the mitochondrial genome of Paracondylactis sinensis, with 13 protein coding genes, one ORF, 2 tRNAs, and 2 rRNAs.
The species name “Cereus sinensis” was frequently used for the edible sea anemone under study in many Chinese reports (Wu et al. Citation2011a–d). However, the identity of this species has been diagnosed as Paracondylactis sinensis of the family Actiniidae by Chinese taxonomists based on morphological characters (Pei Citation1998; Li Citation2013; Li and Xu Citation2020). A caution should be noted is that the name “Cereus sinensis” has been obsoleted in 2015 and it has been revised as Calliactis sinensis of the family Hormathiidae (Daly et al. Citation2022). Even so, the 120 tentacles in Calliactis sinensis apparently distinguished it from P. sinensis in the present study that has only 96 tentacles (Carlgren Citation1934). Moreover, the phylogenetic tree constructed in this study further confirmed that P. sinensis was in family Actiniidae, whereas the genus Calliactis (represented by C. polypus in the tree) was in family Hormathiidae that was distantly related to P. sinensis. The separation of the two species was supported by high bootstrap values ().
Figure 3. The maximum likelihood tree of Paracondylactis sinensis and other related sea anemones built from the concatenated amino acids of 13 protein coding genes. FLU + F + I + G4 model is used. Numbers near the nodes indicates SH-aLRT and ultrafast bootstrap support values from 20,000 replicates. The species P. sinensis whose sequence was produced in this study is in bold. The families each species belongs to are shown near the species name. The families that need further revision are marked with the symbols of hash.
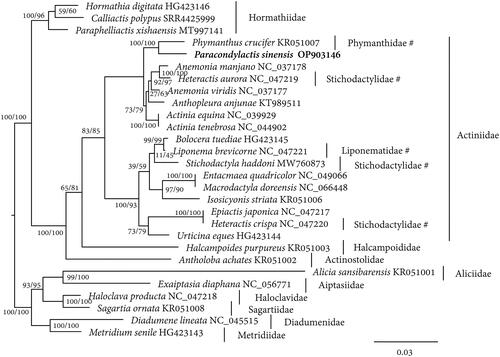
The phylogenetic tree constructed in this study also showed that the members of families Stichodactylidae, Liponematidae, and Phymanthidae are distributed within the family Actiniidae (). This phenomenon indicates that the currently taxonomic relationships of Actiniidae and other related families are entangled. A further revision is called for in future studies to disentangle their confusing relationships.
Authors’ contributions
Junyuan Li designed the project, analyzed data, drafted the paper, and drew the figure. Yang Li identified the species and conceived the project. Tinghui Xie revised the intellectual content and analyzed data. Juan Feng analyzed and interpretated data. Xuyi Yang collected and shipped the sample, and revised it critically for intellectual content. Zifeng Zhan conceived and designed the project and provided the funding. All authors approve of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical statement
Samples were collected from lower tide zones of Taizhou (121°39.5′E, 28°20.1′N) and Wenzhou (121°04.2′E, 27°27.9′N) cities during ebb tide. Approval number for access to field sites and experiments is JJZB-PYCG-2021112901 from Nanji Islands National Marine Nature Reserve Administration. The experiment in this study complied with the International Union for Conservation of Nature (IUCN) policies research involving species at risk of extinction, the convention on biological diversity, and the convention on the trade in endangered species of wild fauna and flora.
Supplemental Material
Download TIFF Image (5.3 MB)Acknowledgments
Thanks are given to the Center for High Performance Computing and System Simulation, Pilot National Laboratory for Marine Science and Technology (Qingdao) and Oceanographic Data Center, IOCAS, for providing computing power.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under the accession no. OP903146. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA934839, SRR23454370, and SAMN33284299, respectively.
Additional information
Funding
References
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19(5):455–477.
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319. doi: 10.1016/j.ympev.2012.08.023.
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30(15):2114–2120. doi: 10.1093/bioinformatics/btu170.
- Carlgren O. 1934. Zur Revision der Actiniarien. Arkiv Für Zoologi. 26A(18):1–36.
- Chernomor O, von Haeseler A, Minh BQ. 2016. Terrace aware data structure for phylogenomic inference from supermatrices. Syst Biol. 65(6):997–1008. doi: 10.1093/sysbio/syw037.
- Chi SI, Urbarova I, Johansen SD. 2018. Expression of homing endonuclease gene and insertion-like element in sea anemone mitochondrial genomes: lesson learned from Anemonia viridis. Gene. 652:78–86. doi: 10.1016/j.gene.2018.01.067.
- Cong H, Lei Y, Kong L. 2020. The mitochondrial genome of the orange-striped green sea anemone Diadumene lineata (Actiniaria: Diadumenidae): the first complete sequence in the family Diadumenidae. Mitochondrial DNA B Resour. 5(1):591–592. doi: 10.1080/23802359.2019.1710594.
- Daly M, Fautin D, Rodríguez E. 2022. World list of Actiniaria; [accessed 2022 Nov 28]. https://www.marinespecies.org/actiniaria on.
- Emblem Å, Okkenhaug S, Weiss ES, Denver DR, Karlsen BO, Moum T, Johansen SD. 2014. Sea anemones possess dynamic mitogenome structures. Mol Phylogenet Evol. 75:184–193.
- Feng C, Liu R, Xu W, Zhou Y, Zhu C, Liu J, Wu B, Li Y, Qiu Q, He S, et al. 2021. The genome of a new anemone species (Actiniaria: Hormathiidae) provides insights into deep-sea adaptation. Deep Sea Res Part I. 170:103492. doi: 10.1016/j.dsr.2021.103492.
- Foox J, Brugler M, Siddall ME, Rodríguez E. 2016. Multiplexed pyrosequencing of nine sea anemone (Cnidaria: Anthozoa: Hexacorallia: Actiniaria) mitochondrial genomes. Mitochondrial DNA A DNA Mapp Seq Anal. 27(4):2826–2832. doi: 10.3109/19401736.2015.1053114.
- Huang TY. 2009. Complete mitochondrial DNA sequence analyses of the sea anemones Mesactinia genesis and Heteractis aurora as well as the sea squirt Eudistoma gilboviride of Taiwan. https://books.google.com.sg/books/about/Complete_Mitochondrial_DNA_Sequence_Anal.html?id=rUYfygAACAAJ&redir_esc=y.
- Johansen SD, Chi SI, Dubin A, Jørgensen TE. 2021. The mitochondrial genome of the sea anemone Stichodactyla haddoni reveals catalytic introns, insertion-like element, and unexpected phylogeny. Life. 11(5):402. doi: 10.3390/life11050402.
- Li JY, Liao YW, Li J, He LS. 2019a. The complete mitochondrial genome of the deep-sea amphipod Eurythenes magellanicus (Crustacea: Amphipoda: Lysianassidae). Mitochondrial DNA B Resour. 5(1):337–339. doi: 10.1080/23802359.2019.1703573.
- Li JY, Song ZL, Yan GY, He LS. 2019b. The complete mitochondrial genome of the largest amphipod, Alicella gigantea: insight into its phylogenetic relationships and deep sea adaptive characters. Int J Biol Macromol. 141:570–577. doi: 10.1016/j.ijbiomac.2019.09.050.
- Li Y. 2013. Species Composition and faunastic characteristics of the order Actiniaria (Cnidaria: Anthozoa) in Chinese waters. Dissertation of Institute of Oceanology, Chinese Academy of Sciences, Qingdao, 201. (in Chinese).
- Li Y, Xu KD. 2020. Species diversity and faunal characteristics of the order Actiniaria (Cnidaria: Anthozoa) in the seas of China. Oceanol Limnol Sin. 51(3):434–443. (in Chinese).
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood Phylogenies. Mol Biol Evol. 32(1):268–274. doi: 10.1093/molbev/msu300.
- Ni Y, Li JL, Zhang C, Liu C. 2023. Generating sequencing depth and coverage map for organelle genomes doi: 10.17504/protocols.io.4r3l27jkxg1y/v1.
- Pei ZN. 1998. Fauna Sinensis. Coelenterata: Actiniaria, Ceriantharia, Zoanthidea. Beijing: Science Press; p. 286. (in Chinese).
- Stewart ZK, Pavasovic A, Hock DH, Prentis PJ. 2017. Transcriptomic investigation of wound healing and regeneration in the cnidarian Calliactis polypus. Sci Rep. 7(1):41458. doi: 10.1038/srep41458.
- Surm JM, Stewart ZK, Papanicolaou A, Pavasovic A, Prentis PJ. 2019. The draft genome of Actinia tenebrosa reveals insights into toxin evolution. Ecol Evol. 9(19):11314–11328. doi: 10.1002/ece3.5633.
- Wilding CS, Weedall GD. 2019. Morphotypes of the common beadlet anemone Actinia equina (L.) are genetically distinct. J Exp Mar Biol Ecol. 510:81–85. doi: 10.1016/j.jembe.2018.10.001.
- Wu JP, Yang HP, Liu HL. 2011a. Artificial propagation experiment of Cereus sinensis Verrill, a Chinese sea anemone (Part I). Aquac Sci. 259(3):70–71. (in Chinese)
- Wu JP, Yang HP, Liu HL, Zhang ZY. 2011b. Artificial propagation experiment of Cereus sinensis Verrill, a Chinese sea anemone (Part II). Aquac Sci. 260(4):72–74. (in Chinese)
- Wu JP, Yang HP, Liu HL, Zhang ZY. 2011c. Preliminary experiment on parental domestication of Cereus sinensis Verrill, a Chinese sea anemone. J Aquac. 32(04):47–49. (in Chinese)
- Wu JP, Yang HP, Liu HL, Zhang ZY, Zhong JS, Wang JJ. 2011d. Preliminary study on early development of Cereus sinensis Verrill, a Chinese sea anemone. J Shanghai Ocean Univ. 20(6):826–830. (in Chinese)
- Zhang D, Gao F, Jakovlić I, Zou H, Zhang J, Li WX, Wang GT. 2020. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour. 20(1):348–355. doi: 10.1111/1755-0998.13096.
- Zhang L, Zhu Q. 2017. Complete mitochondrial genome of the sea anemone, Anthopleura midori (Actiniaria: Actiniidae). Mitochondrial DNA A DNA Mapp Seq Anal. 28(3):335–336. doi: 10.3109/19401736.2015.1122770.

