Abstract
Batocera rubus severely impacts on the health of banyan trees. In this study, the whole mitochondrial genome for B. rubus was found to be 16,158 bp with a GC content of 23.9%, including 39.1% A, 37.0% T, 14.8% C, and 9.1% G. This genome contains 13 protein-coding genes, 22 tRNAs, and two rRNAs. Phylogenetic analysis revealed that B. rubus is close to Batocera celebiana. This study provides valuable information that can help improve the classification and phylogeny of B. rubus and facilitate further evolutionary studies.
Introduction
Batocera rubus Linnaeus 1785 (Coleoptera: Cerambycidae) is widely distributed beetle species found in China, India, South Korea, and many other countries (Liu et al. Citation2003). Due to their large size and long life cycle, the damage caused by Batocera rubus to trees is often relatively large. The larvae feed on the phloem and xylem of plants affecting their growth and leading to the loss of the affected branches, stems, and even the entire plant (Liu et al. Citation2003). B. rubus has a severe economic impact in forestry industry; however, there is a paucity of research on B. rubus that limits our understanding as well as the prevention and control of this pest. To better understand and reveal the genetic and evolutionary familiarity of B. rubus, the whole mitochondrial genome of B. rubus was sequenced and assembled. The genome of B. rubus has been published and can be retrieved from GenBank (BioProject: PRJNA796453; Bio-Sample: SAMN24860787; SRA: SRR17574369).
Materials and methods
Batocera rubus adults were trapped by using the trapping method in Minhou, Fujian Province, China (118°57′21″E, 26°9′19″N). Adults are medium-sized yellow-brown beetles (Hemadri and Reddy Citation2019). Batocera rubus has a kidney-shaped spot marking on their pronotum as well as white or rose-red colors (). There are four white spots on each elytron; the fourth spot is the smallest and the second spot is the largest. There are often one or two small spots that are higher than the second spot and are sometimes coupled to the second spot. The spots on the elytra are arranged in a longitudinal line along the midline. The bottom of the elytra features a small and dense granular protuberance that occupies approximately 1/4 of the elytra. The insect samples were kept with a TN-202101 voucher at the Key Laboratory of Integrated Pest Management in Ecological Forests, Fujian Agriculture and Forestry University.
Figure 1. Female of Batocera rubus Linnaeus, 1785, dorsal view. Scale = 1 cm. This is an original image by the authors.

Total genomic DNA from a single adult legs was extracted by using the TruSeq DNA Sample Preparation kit (Vanzyme, Nanjing, China), and the DNA concentration was determined using a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA). A DNA library was constructed using the transposase method and the library was prepared with randomly interrupted 300-bp fragments. The constructed library was sequenced with 150-bp pair-end reads on the Illumina Hiseq2500 platform (Illumina, San Diego, CA). Clean reads were assembled using MitoZ and metaSPAdes (Nurk et al. Citation2017). Annotation was performed using GeSeq (Kearse et al. Citation2012; Guyeux et al. Citation2019) and plotted on a gene map using the ogdraw software (Greiner et al. Citation2019). The nucleotide sequences of different insect species were downloaded from the NCBI website (https://www.ncbi.nlm.nih.gov/) and used for phylogenetic analysis. An evolutionary tree containing the COI gene of 17 different species via MEGA 7.0 was constructed (Górecki et al. Citation2011; Kumar et al. Citation2016) using the maximum-likelihood statistical method and the Kimura 2-parameter model with 1000 bootstrap replicates.
Results
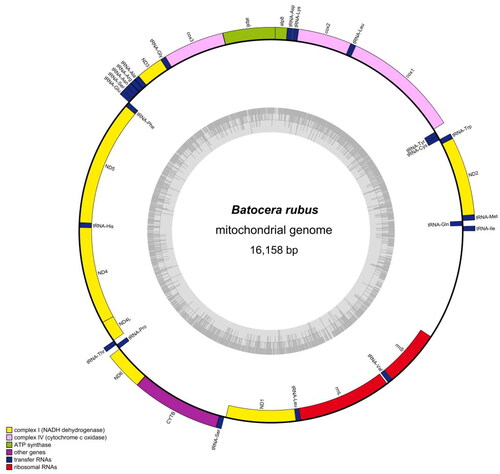
A total of 46,027,082 clean reads were obtained out of 47,766,884 raw reads. The complete mitogenome of B. rubus is sequenced to be 16,158 bp in length with an average depth of 3073.49X (Supplementary Figure 1). In addition, the Genebook of B. rubus revealed that the overall mitochondrial genome of B. rubus contains 13 PCGs (), which encode 3677 amino acids. Nine PCGs (ATP6, ATP8, COX1, COX2, COX3, CYTB, ND2, ND3, and ND6) were coded clockwise, and four PCGs (ND1, ND4, ND4L, and ND5) were coded counterclockwise. The gene length of rrnS was 780 bp, and that of rrnL was 1294 bp. The genome-wide GC content was 23.90%, of which 39.1% was A, 14.8% C, 9.1% G, and 37.0% T.
Figure 2. Complete mitochondrial genome map of Batocera rubus. The arrangement of 37 genes is represented in the map, including 13 protein coding genes, 22 tRNA genes, and two rRNA genes.
The evolutionary tree included 13 Cerambycidae (Monochamus alternatus, Anoplophora chinensis, Anoplophora glabripennis, Psacothea hilaris, Paraglenea fortunei, Anoplophora horsfieldii, Blepephaeus succinctor, Apriona rugicollis, Batocera horsfieldi, Batocera celebiana, Apriona swainsoni, Oberea nigriventris, and Acanthocinus griseus), two Nitidulidae (Omosita colon and Meligethes aeneus), and one Aseminae (Arhopalus unicolor) ().
Figure 3. Maximum-likelihood tree of Batocera rubus related to 16 different species of Coleoptera based on the COI gene. Bootstrap support values are labeled near the branch.
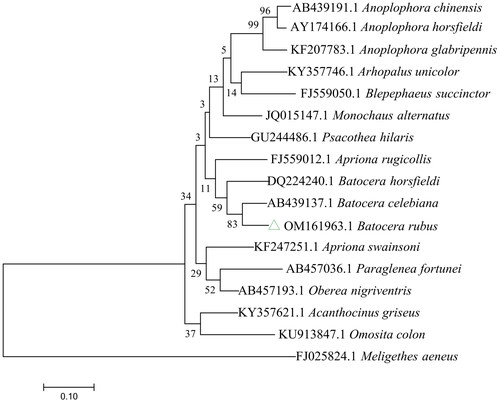
The phylogenetic tree revealed that B. rubus constituted a monophyletic group with 14 other Cerambycidae species using the entire mitochondrial genome of Meligethes aeneus as an outgroup. Additionally, phylogenetic tree analysis showed that B. rubus and B. celebiana formed a monophyletic clade with a high support value (BS = 83%), and these species showed a close relationship. In addition to providing genetic information that can be used for the genus Batocera, the entire mitochondrial genome of B. rubus will be helpful for studying the evolutionary traits of Batocera ().
Table 1. Information on the mitogenomes of the species used in this study
Discussion
In recent decades, the unique features of the insect mitochondrial genome have led to a wide range of applications in phylogenetic and population genetic studies at different levels of population genetics, molecular evolution, comparative and evolutionary genomics, etc. (Galtier et al. Citation2009; Cameron Citation2014). In this study, the whole mitochondrial genome of B. rubus was successfully sequenced and assembled, which assists in the further improvement of the taxonomy and phylogeny of B. rubus. Comparison of the mitochondrial genomes of B. rubus and B. celebiana revealed that the GC content of these species was essentially the same. In addition, the percentages of each nucleobase were the same, which may be related to the fact that they have the closest relatives in the evolutionary tree. The present results provide important information for studying the evolution of the recombinant mitochondrial genome of B. rubus and provide a basis for developing strategies for controlling B. rubus.
Author contributions
Conceived and designed the experiments: Songqing-Wu. Performed the experiments: Rong Deng, Bowei Zhou, Yiqi Lin, and Xianyun Lin. Analyzed the data: Yunzhu Sun. Wrote the paper: Rong Deng. All authors are involved: final approval of the version to be published. All authors agreed to take responsibility for all aspects of the work.
Ethical approval
The material involved in the article does not involve ethical conflicts. This study was permitted by the Key Laboratory of Integrated Pest Management in Ecological Forests, FAFU, China. All collection and sequencing work were strictly executed under local legislation and related laboratory regulations to protect wild resources.
Supplemental Material
Download MS Word (802.9 KB)Supplemental Material
Download JPEG Image (791.7 KB)Supplemental Material
Download JPEG Image (485.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data supporting this study’s findings are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov under assessment number OM161963. The associated BioProject, Bio-Sample, and SRA numbers are PRJNA796453, SAMN24860787, and SRR17574369, respectively.
Additional information
Funding
References
- Cameron SL. 2014. Insect mitochondrial genomics: implications for evolution and phylogeny. Annu Rev Entomol. 59(1):95–117. doi: 10.1146/annurev-ento-011613-162007.
- Dai X-Y, Zhang H, Xu X-D, Jia Y-Y, Zhang J-Y, Yu D-N, Cheng H-Y. 2020. The complete mitochondrial genome of Annamanum lunulatum (Coleoptera: Lamiinae) and its phylogeny. Mitochondrial DNA B Resour. 5(1):551–553. doi: 10.1080/23802359.2019.1710284.
- Galtier N, Nabholz B, Glémin S, Hurst GD. 2009. Mitochondrial DNA as a marker of molecular diversity: a reappraisal. Mol Ecol. 18(22):4541–4550. doi: 10.1111/j.1365-294X.2009.04380.x.
- Górecki P, Burleigh GJ, Eulenstein O. 2011. Maximum likelihood models and algorithms for gene tree evolution with duplications and losses. BMC Bioinformatics. 12(Suppl. 1):S15. doi: 10.1186/1471-2105-12-S1-S15.
- Greiner S, Lehwark P, Bock R. 2019. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 47(W1):W59–W64. doi: 10.1093/nar/gkz238.
- Guyeux C, Charr J-C, Tran HTM, Furtado A, Henry RJ, Crouzillat D, Guyot R, Hamon P. 2019. Evaluation of chloroplast genome annotation tools and application to analysis of the evolution of coffee species. PLOS One. 14(6):e0216347. doi: 10.1371/journal.pone.0216347.
- Hemadri T, Reddy DS. 2019. Incidence pattern of stem borer, Batocera rubus in Moringa. Pest Manage Hortic Ecosyst. 24:71–72.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649. doi: 10.1093/bioinformatics/bts199.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33(7):1870–1874. doi: 10.1093/molbev/msw054.
- Kusakabe Y. 2001. An adult host record of Oberea nigriventris (Coleoptera, Cerambycidae). Elytra. 29(1):226.
- Lin MY, Perissinotto R, Clennell L. 2021. Census of the longhorn beetles (Coleoptera, Cerambycidae and Vesperidae) of the Macau SAR, China. Zookeys. 1049:79–161. doi: 10.3897/zookeys.1049.65558.
- Liu DM, Gao ZZ, Xing FW. 2003. Bionomics and control of Batocera rubus. Forest Pest Dis. 22(6):10–12.
- Martikainen P. 2002. Ecology and conservation status of Acanthocinus griseus (Fabricius, 1792) (Coleoptera: Cerambycidae) in Finland. Entomol Fennica. 13(1):41–50. doi: 10.33338/ef.84134.
- McKenna DD, Scully ED, Pauchet Y, Hoover K, Kirsch R, Geib SM, Mitchell RF, Waterhouse RM, Ahn S-J, Arsala D, et al. 2016. Genome of the Asian longhorned beetle (Anoplophora glabripennis), a globally significant invasive species, reveals key functional and evolutionary innovations at the beetle–plant interface. Genome Biol. 17(1):227. doi: 10.1186/s13059-016-1088-8.
- Nurk S, Meleshko D, Korobeynikov A, Pevzner PA. 2017. metaSPAdes: a new versatile metagenomic assembler. Genome Res. 27(5):824–834. doi: 10.1101/gr.213959.116.
- Ohbayashi N, Ogawa J, Su ZH. 2009. Phylogenetic analysis of the lamiine genus Anoplophora and its relatives (Coleoptera, Cerambycidae) based on the mitochondrial COI gene. Spec Bull Coleopterol Soc Japan. 7:309–324.
- Ohbayashi N. 2018. A new subspecies of Anoplophora horsfieldii (Hope, 1842) (Coleoptera: Cerambycidae: Lamiinae). Japan J Syst Entomol. 24(1):39–42.
- Que S, Yu A, Liu P, Jin M, Xie G. 2019. The complete mitochondrial genome of Apriona swainsoni. Mitochondrial DNA Part B. 4(1):931–932.
- Saeb ATM, Grewal PS. 2014. Phylogenetic and population genetic structure of the yellow spotted longicorn beetle Psacothea hilaris. Adv Life Sci. 2:56–67.
- Smart LE, Blight ME. 2000. Response of the pollen beetle, Meligethes aeneus, to traps baited with volatiles from oilseed rape, Brassica napus. J Chem Ecol. 26(4):1051–1064. doi: 10.1023/A:1005493100165.
- Tsubaki R, Hosoda N, Kitajima H, Takanashi T. 2014. Substrate-borne vibrations induce behavioral responses in the leaf-dwelling cerambycid, Paraglenea fortunei. Zoolog Sci. 31(12):789–794. doi: 10.2108/zs140029.
- Wang J, Hu P, Gao P, Tao J, Luo Y. 2017. Antennal transcriptome analysis and expression profiles of olfactory genes in Anoplophora chinensis. Sci Rep. 7(1):15470. doi: 10.1038/s41598-017-15425-2.
- Wang Y, Wang M, Hu G, Xu W, Wang Y, Wang J. 2020. Temperature-dependent development of Omosita colon at constant temperature and its implication for PMImin estimation. J Forensic Leg Med. 72:101946. doi: 10.1016/j.jflm.2020.101946.
- Wen J, Li Y, Huang Z, Ma J, Wang S, Wu S. 2021. The complete mitochondrial genome of Arhopalus unicolor (Coleoptera: Cerambycidae). Mitochondrial DNA B Resour. 6(2):596–597. doi: 10.1080/23802359.2021.1875923.
- Yang H, Cai Y, Zhuo Z, Yang W, Yang C, Zhang J, Yang Y, Wang B, Guan F. 2018. Transcriptome analysis in different developmental stages of Batocera horsfieldi (Coleoptera: Cerambycidae) and comparison of candidate olfactory genes. PLoS ONE. 13(2):e0192730 10.1371/journal.pone.0192730.
- Zhou J, Yu H-Y, Zhang W, Ahmad F, Hu S-N, Zhao L-L, Zou Z, Sun J-H. 2018. Comparative analysis of the Monochamus alternatus immune system. Insect Sci. 25(4):581–603. doi: 10.1111/1744-7917.12453.
