Abstract
In this study, we report the novel, complete mitochondrial genomes of two dwarf African antelope species: Raphicerus melanotis (Cape grysbok) and R. sharpei (Sharpe’s grysbok). The circular mitogenomes were 16,384 and 16,392 base pairs in length, respectively, and each contained the expected 37 genes typically found in mammalian mitogenomes. The maximum-likelihood phylogenetic analysis placed R. sharpei as the sister lineage to R. campestris, known as steenbok, which is the only other member of the Raphicerus genus, with 83% bootstrap support, to the exclusion of R. melanotis (100% bootstrap support). This corroborated previous findings based on the cytochrome b gene only. The number of base differences per site between the coding regions of the mitogenomes of R. sharpei and R. campestris was 0.0519, while it was 0.0701 between R. sharpei and R. melanotis and 0.0709 between R. melanotis and R. campestris. The novel grysbok mitogenomes will be valuable resources in future phylogenetic analyses, and phylogeographic and conservation genetics studies.
Introduction
The genus Raphicerus (Family: Bovidae) consists of small (∼10 kg), predominantly browsing, dwarf antelope species, occurring from eastern to southern Africa (). Three species are recognized: Raphicerus melanotis Thunberg 1811 (Cape grysbok), Raphicerus sharpei Thomas 1897 (Sharpe’s grysbok), and Raphicerus campestris Thunberg 1811 (steenbok). The two grysbok species are allopatric, while the steenbok is sympatric with both grysbok species for parts of its range. While all three species are currently classified as Least Concern on the IUCN Red List of Threatened Species, there is a “continuing decline in area, extent and/or quality of habitat” for all three species (IUCN Citation2016a, Citation2016b; Palmer et al. Citation2017). Only R. campestris has available whole nuclear (Chen et al. Citation2019) and mitochondrial genome (mitogenome) data (Hassanin et al. Citation2012). Raphicerus melanotis and R. sharpei have only one complete mitochondrial gene (cytochrome b) and a few partial mitochondrial and nuclear gene sequences available (Matthee and Robinson Citation1999; Bärmann et al. Citation2013). No population-level genetic studies have been conducted on any of the three species.
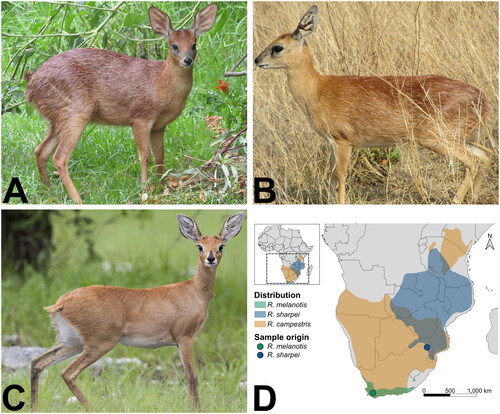
Figure 1. Photographs and range map of the three Raphicerus species. A R. melanotis (Cape grysbok) female in Robertson, Western Cape Province, South Africa (©Barbara Claassen), B R. sharpei (Sharpe’s grysbok) female in Kruger National Park, South Africa (©Paulette Bloomer), C R. campestris (steenbok) female in Etosha National Park, Namibia (by Yathin S. Krishnappa - Own work, CC by-SA 3.0, https://creativecommons.org/licenses/by-sa/3.0/, https://commons.wikimedia.org/w/index.php?curid=24567649), included for comparative purposes. The original photo was cropped for this figure. Both grysbok species have conspicuous white hairs scattered throughout their coat, which are absent in steenbok. D Distribution range map of the three Raphicerus species. Distribution data were obtained from the IUCN Red List page for each species (IUCN (International Union for Conservation of Nature) Citation2017, Citation2008a, Citation2008b). the map was generated in QGIS v3.22.2 (https://qgis.org/en/site/).
Here, we present the novel, complete mitogenomes of R. melanotis and R. sharpei, assembled from shotgun sequencing reads, and compare these to the available R. campestris mitogenome (Hassanin et al. Citation2012). The mitogenomes will be valuable in future investigations of the evolutionary history of the genus, and phylogeographic and conservation genetics studies of the grysbok species.
Materials and methods
The R. melanotis sample was heart tissue (from a carcass) donated by CapeNature and originated from the Western Cape Province, South Africa (latitude: −33.709, longitude: 19.493, ). The carcass was morphologically identified as R. melanotis based on the white hairs interspersed in its overall rufous coat, which distinguishes it from the sympatric R. campestris that lacks the interspersed white hairs, while its sampling location distinguished it from the allopatric R. sharpei () (Castley and Lloyd Citation2013). The sample was deposited at the South African National Biodiversity Institute (SANBI) National Zoological Gardens (NZG) Biobank, Pretoria (https://www.sanbi.org/, Kim Labuschagne: [email protected]) under the voucher number 85533. The carcass was deposited at the Iziko Museums of South Africa Terrestrial Vertebrates Collection, Cape Town (https://www.iziko.org.za/, Jofred Opperman: [email protected]) under the voucher number ZM-042619.
The R. sharpei sample was a piece of dry skin obtained from a taxidermist. The specimen was morphologically identified as R. sharpei based on the white hairs interspersed in its reddish-fawn coat, which distinguishes it from the sympatric R. campestris that lacks such hairs, while its sampling location distinguished it from the allopatric R. melanotis () (Hoffman and Wilson Citation2013). The origin of the animal (which was not explicitly sampled for this study) is near Mica, Limpopo Province, South Africa (latitude: −24.1, longitude: 30.8, ). The sample was deposited at the SANBI NZG Biobank under voucher number 85526.
Genomic DNA was isolated from heart tissue using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) following the tissue spin column protocol (including RNase A treatment). The user-developed “Purification of DNA from nails, hair, or feathers using DNeasy Blood & Tissue Kit (DY04 Aug-06)” (https://www.qiagen.com/dk/resources/resourcedetail?id=a5a065dc-e287-4a61-b917-9792e25ab42f&lang=en) was used (including RNase A treatment) to isolate genomic DNA from the skin sample.
PCR-free libraries were constructed from the genomic DNA with Illumina TruSeq adapters, 350 base pair (bp) inserts, and sequenced on an Illumina NovaSeq 6000 (Illumina, San Diego, USA) with 150 bp paired-end reads, by Novogene UK. Adapters were removed and low-quality reads were filtered using fastp v0.23.2 (Chen et al. Citation2018). Because the sequencing data generated was for high-coverage nuclear genome sequencing, the clean, paired reads were randomly downsampled for the mitogenome assembly to 5 gigabases using BBmap v39.01 (Bushnell Citation2022) (command: reformat.sh samplebasestarget = 5000000000). NOVOPlasty v4.3.1 (Dierckxsens et al. Citation2017) was used to assemble the mitogenomes from the downsampled reads with a kmer of 33, and using as seed the available R. campestris reference mitogenome (JN632693.1) (Hassanin et al. Citation2012). The two assembled mitogenomes were annotated using MITOS2 (Donath et al. Citation2019). Annotations were manually curated by comparison to the R. campestris mitogenome in Geneious Prime v2023.0.4 (http://www.geneious.com/). The naming convention used by MITOS2 was retained (Bernt et al. Citation2013).
A maximum-likelihood phylogenetic tree was constructed in IQ-TREE v2.2.0 (Minh et al. Citation2020), using concatenated MAFFT (Katoh and Standley Citation2013) alignments of all genes encoded on the mitogenomes (total of 15,431 bp) of the three Raphicerus species, all publicly available mitogenomes from the sister clade of Raphicerus, i.e. Dorcatragus+Madoqua (Hassanin et al. Citation2012), and Aepyceros melampus (impala) used as the outgroup, as it is in the same Family (Bovidae), but a different tribe (Aepycerotini) than the other species (Antilopini) () (Hassanin et al. Citation2012). The alignment was divided into three partitions: protein-coding genes, rRNA genes, and tRNA genes. ModelFinder Plus (Kalyaanamoorthy et al. Citation2017) is embedded in IQ-TREE and was used to determine the best partition scheme and find the best model for each partition. The optimal partition scheme was to combine the rRNA and tRNA genes into a single partition, with the protein-coding genes as a separate partition (Chernomor et al. Citation2016). The best-fit DNA substitution model for the rRNA-tRNA partition was determined to be TIM2+F+R2 based on the Bayesian Information Criterion (BIC), while the best-fit codon model for the protein-coding genes was determined to be GY+F+R3 based on the BIC. The phylogenetic tree was subsequently estimated in IQ-TREE with two independent runs, each with 1000 ultrafast bootstrap replicates (Hoang et al. Citation2018). Evolutionary divergence (the number of base differences per site between sequences) of the three Raphicerus mitogenomes was estimated using the p-distance method in MEGA v11 (Tamura et al. Citation2021) with the same dataset that was used in the phylogenetic analysis, with rate variation among sites modeled with a gamma distribution (shape parameter = 1) and 100 bootstrap replicates to estimate the standard error (SE).
All scripts and configuration files used in the analyses are available at: https://github.com/DeondeJager/Raphicerus_mitogenomes.
Results
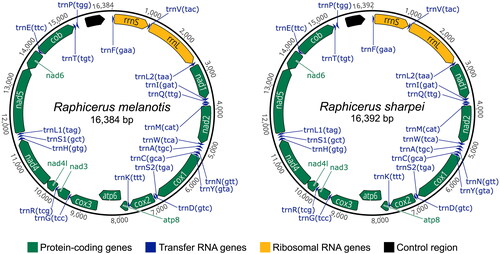
The R. melanotis and R. sharpei mitogenomes were each assembled into a single contig with mean depth-of-coverage of 3588X and 154X, respectively (Figure S1). GC content of the R. melanotis mitogenome was 38.3% and R. sharpei was 38.5%, which were comparable to that of the R. campestris mitogenome (38.3%). The R. melanotis mitogenome (16,384 bp) was 8 bp shorter than the R. sharpei and R. campestris mitogenomes (16,392 bp). Each grysbok mitogenome contained the expected 37 genes, in the same order as in R. campestris: 13 protein-coding genes (nad1-6, nad4l, cox1-3, atp6, atp8, and cob), two rRNA genes (12S: rrnS and 16S: rrnL), and 22 tRNA genes (). The control region of R. sharpei was the same length as R. campestris (963 bp), with that of R. melanotis being shorter at 955 bp.
Figure 2. Mitochondrial genome maps of R. melanotis (Cape grysbok) and R. sharpei (Sharpe’s grysbok) produced in Geneious Prime and further edited in Inkscape v1.2 (https://inkscape.org/).
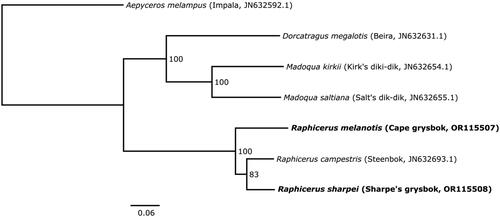
The phylogenetic analysis placed R. sharpei and R. campestris as sister lineages, with 83% bootstrap support, to the exclusion of R. melanotis (). The number of base differences per site between the coding regions of the mitogenomes were as follows: [sharpei-campestris] = 0.0519 (SE = 0.0020); [sharpei-melanotis] = 0.0701 (SE = 0.0022), [melanotis-campestris] = 0.0709 (SE = 0.0023).
Figure 3. Consensus maximum-likelihood phylogenetic tree of the concatenated mitogenome coding regions (15,431 bp) of Raphicerus, Madoqua and Dorcatragus species, with Aepyceros melampus (impala) as outgroup. The sequences generated for this study are shown in bold. Values at nodes show bootstrap support for each branch from 1000 replicates. The tree was estimated in IQ-TREE and visualized in FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/). Common names and GenBank accession numbers are indicated in parentheses. The following sequences were used: Raphicerus melanotis OR115507 (this study), Raphicerus sharpei OR115508 (this study), Raphicerus campestris JN632693.1 (Hassanin et al. Citation2012), Madoqua kirkii JN632654.1 (Hassanin et al. Citation2012), Madoqua saltiana JN632655.1 (Hassanin et al. Citation2012), Dorcatragus megalotis JN632631.1 (Hassanin et al. Citation2012), and Aepyceros melampus JN632592.1 (Hassanin et al. Citation2012).
Discussion and conclusion
We assembled the complete mitogenomes of Raphicerus melanotis and R. sharpei from shotgun sequencing reads. We observed 23-fold higher coverage of the mitogenome for R. melanotis compared to R. sharpei, which likely reflects the high copy number of mitochondria (the powerhouse of the cell) in heart tissue compared to skin, as the same amount of sequence data was used for each assembly.
Morphologically, R. melanotis, and R. sharpei are assumed to be sister species based on the white hairs scattered through their coats (absent in R. campestris), and their having shorter legs and broader mouths compared to R. campestris (Du Toit, Citation2005; Groves and Grubb Citation2011; Castley and Lloyd Citation2013). Our mitogenome phylogeny did not show them as sister species. Rather, our analysis supports previous findings (based on cytochrome b) that R. sharpei and R. campestris are sister species at the mitochondrial level (Matthee and Robinson Citation1999). However, a consensus tree of eight genes (four mitochondrial and four nuclear) supported R. melanotis and R. sharpei as sister species to the exclusion of R. campestris (Bärmann et al. Citation2013), as would be expected based on morphology.
These contradictory findings are indicative of a complex evolutionary history within the genus, with incomplete lineage sorting and/or ancestral gene flow playing an important role in the present-day genetic relationships of the species. The grysbok mitogenomes provide a valuable resource for future phylogenetic studies of Bovidae, as well as phylogeographic, conservation genetics, and ancient DNA studies of these species.
Ethical approval
Ethical clearance was obtained from the European Research Executive Agency (call reference: H2020-MSCA-IF-2020, proposal number: 101026951).
CapeNature seized a Raphicerus melanotis sub-adult male carcass in an illegal snaring case on 24 June 2022 on the R43 road Worcester to Villiersdorp near Klipkrans Game Farm, Western Cape Province, South Africa. The case was finalized and the carcass was forfeited to the State (CapeNature), after which heart tissue was sampled and donated to DdJ for research purposes. DdJ has the donation letter. Raphicerus sharpei is listed as a Threatened or Protected Species (ToPS) in South Africa under the National Environmental Management: Biodiversity Act, Citation2004 (Act 10 of 2004): Amendment of Critically Endangered, Endangered, Vulnerable and Protected Species List (Government Gazette, No. 30568, 14 December 2007). Consequently, the appropriate ToPS permit was obtained to have the sample for research purposes from the Gauteng Department of Agriculture and Rural Development, as Nico van Rooyen Taxidermy (where the sample was obtained) is located in Gauteng. The ToPS permit number is 0002558 and the Department of Agriculture, Land Reform, and Rural Development Section 20 permit number is 12/11/1/1/8/MG (2102). Neither R. melanotis nor R. sharpei are listed under CITES.
Authors’ contributions
DdJ and EDL were involved in conception and design. DdJ was involved in sample collection, performed the analyses and interpretation of the results, and drafted the manuscript. EDL revised the manuscript critically for intellectual content. Both authors were involved in the final approval of the version to be published. Both authors agree to be accountable for all aspects of the work.
Supplemental Material
Download JPEG Image (3.2 MB)Supplemental Material
Download MS Word (12.8 KB)Acknowledgements
The authors thank Corne Claassen of CapeNature for providing the R. melanotis sample and Nico van Rooyen Taxidermy for providing the R. sharpei sample, as well as Kim Labuschagne of SANBI NZG and Jofred Opperman of Iziko Museums of South Africa for accepting the donated samples into the respective collections. The authors also thank Anri van Wyk for extracting the DNA from the R. melanotis sample.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/ under the accession no. OR115507-OR115508. The associated BioProject, SRA, and BioSample numbers are PRJNA982611, SRR24893559-SRR24893560, and SAMN35711614-SAMN35711615, respectively.
Additional information
Funding
References
- Bärmann EV, Rössner GE, Wörheide G. 2013. A revised phylogeny of Antilopini (Bovidae, Artiodactyla) using combined mitochondrial and nuclear genes. Mol Phylogenet Evol. 67(2):484–493. doi:10.1016/j.ympev.2013.02.015.
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319. doi:10.1016/j.ympev.2012.08.023.
- Bushnell B. 2022. July 15). BBMap. SourceForge. https://sourceforge.net/projects/bbmap/.
- Castley G, Lloyd P. 2013. Raphicerus melanotis Cape grysbok. In: J. Kingdon & M. Hoffman editors. Mammals of Africa, Volume VI: pigs, Hippopotamuses, Chevrotain, Giraffes, Deer and Bovids. London: Bloomsbury Publishing; p.304–307.
- Chen L, Qiu Q, Jiang Y, Wang K, Lin Z, Li Z, Bibi F, Yang Y, Wang J, Nie W, et al. 2019. Large-scale ruminant genome sequencing provides insights into their evolution and distinct traits. Science. 364(6446):eaav6202. doi:10.1126/science.aav6202.
- Chen S, Zhou Y, Chen Y, Gu J. 2018. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 34(17):i884–i890. doi:10.1093/bioinformatics/bty560.
- Chernomor O, von Haeseler A, Minh BQ. 2016. Terrace aware data structure for phylogenomic inference from supermatrices. Syst Biol. 65(6):997–1008. doi:10.1093/sysbio/syw037.
- Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, Whitwham A, Keane T, McCarthy SA, Davies RM, et al. 2021. Twelve years of SAMtools and BCFtools. Gigascience. 10(2):giab008. doi:10.1093/gigascience/giab008.
- Dierckxsens N, Mardulyn P, Smits G. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45(4):e18–e18. doi:10.1093/nar/gkw955.
- Donath A, Jühling F, Al-Arab M, Bernhart SH, Reinhardt F, Stadler PF, Middendorf M, Bernt M. 2019. Improved annotation of protein-coding genes boundaries in metazoan mitochondrial genomes. Nucleic Acids Res. 47(20):10543–10552. doi:10.1093/nar/gkz833.
- Du Toit JT, Chimimba CT, Skinner JD. 2005. Order RUMINANTIA. In: C. T. Chimimba, J. D. Skinner, editors, The Mammals of the Southern African Sub-region. 3rd ed., Cambridge: Cambridge University Press; p. 616–714. doi:10.1017/CBO9781107340992.023.
- Groves C, Grubb P. 2011. Ungulate taxonomy. Baltimore: The Johns Hopkins University Press.
- Hassanin A, Delsuc F, Ropiquet A, Hammer C, van Vuuren BJ, Matthee C, Ruiz-Garcia M, Catzeflis F, Areskoug V, Nguyen TT, et al. 2012. Pattern and timing of diversification of Cetartiodactyla (Mammalia, Laurasiatheria), as revealed by a comprehensive analysis of mitochondrial genomes. C R Biol. 335(1):32–50. doi:10.1016/j.crvi.2011.11.002.
- Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the Ultrafast Bootstrap Approximation. Mol Biol Evol. 35(2):518–522. doi:10.1093/molbev/msx281.
- Hoffman M, Wilson VJ. 2013. Raphicerus sharpei Sharpe’s grysbok. In: J. Kingdon & M. Hoffman editors. Mammals of Africa, Volume VI: pigs, Hippopotamuses, Chevrotain, Giraffes, Deer and Bovids. London: Bloomsbury Publishing; p. 308–310.
- IUCN (International Union for Conservation of Nature). 2017. Raphicerus melanotis. The IUCN Red List of Threatened Species. Version 2021-1. https://www.iucnredlist.org. Downloaded on 24/08/2021.
- IUCN. 2016a. IUCN SSC Antelope Specialist Group. 2016. Raphicerus campestris. The IUCN Red List of Threatened Species 2016: e.T19308A50193533. doi:10.2305/IUCN.UK.2016-1.RLTS.T19308A50193533.en.
- IUCN. 2016b. IUCN SSC Antelope Specialist Group. 2016. Raphicerus sharpei. The IUCN Red List of Threatened Species 2016: e.T19307A50193414. doi:10.2305/IUCN.UK.2016-1.RLTS.T19307A50193414.en.
- IUCN. (International Union for Conservation of Nature) 2008a. Raphicerus sharpei. The IUCN Red List of Threatened Species. Version 2021-1. https://www.iucnredlist.org. Downloaded on 24/08/2021.
- IUCN. (International Union for Conservation of Nature) 2008b. Raphicerus campestris. The IUCN Red List of Threatened Species. Version 2021-1. https://www.iucnredlist.org. Downloaded on 27/08/2021.
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 14(6):587–589. doi:10.1038/nmeth.4285.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software Version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780. doi:10.1093/molbev/mst010.
- Matthee CA, Robinson TJ. 1999. Cytochrome b phylogeny of the family Bovidae: resolution within the Alcelaphini, Antilopini, Neotragini, and Tragelaphini. Mol Phylogenet Evol. 12(1):31–46. doi:10.1006/mpev.1998.0573.
- Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R. 2020. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 37(5):1530–1534. doi:10.1093/molbev/msaa015.
- National Environmental Management: Biodiversity Act. 2004. (Act 10 of 2004): Amendment of Critically Endangered, Endangered, Vulnerable and Protected Species List (Government Gazette, No. 30568, 14 December 2007). https://www.gov.za/sites/default/files/gcis_document/201409/305681187.pdf.
- Palmer G, Birss C, Kerley G, Feely J, Peinke D, Castley G. 2017. Raphicerus melanotis. The IUCN Red List of Threatened Species 2017: e.T19306A50193334. doi:10.2305/IUCN.UK.2017-2.RLTS.T19306A50193334.en.
- R Core Team. 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org/.
- Tamura K, Stecher G, Kumar S. 2021. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 38(7):3022–3027. doi:10.1093/molbev/msab120.
- Wickham H. 2016. ggplot2: elegant graphics for data analysis. New York: Springer.
