Abstract
The amphipod Cyphocaris challengeri is a globally distributed, highly abundant species of zooplankton. Here, we report the complete mitochondrial genome of C. challengeri obtained using the Illumina sequencing platform from a specimen collected from Puget Sound, Washington. The mitogenome is a circular DNA molecule with a size of 14,338 bp and 26.7% GC content, with 13 protein-encoding genes, 2 rRNAs, and 22 tRNAs annotated. A maximum likelihood phylogenetic analysis including C. challengeri and all other available mitogenomes from Amphipoda places our mitogenome firmly within the Lysianassoidea superfamily, as expected. The newly described mitochondrial genome of C. challengeri fills a gap in valuable reference data for detecting this organism using molecular methods such as environmental DNA.
Introduction
The genus Cyphocaris Boeck Citation1871 includes pelagic and demersal amphipods with a cosmopolitan distribution (Lowry and Stoddart Citation2003). Twenty species are recognized within this genus (WoRMS Editorial Board Citation2022), including Cyphocaris challengeri Stebbing Citation1888, which is distributed worldwide (Bowman and McCain Citation1967). Cyphocaris challengeri is the most abundant gammarid amphipod in the mesopelagic zone of the subarctic Pacific, and the species plays an important role in the pelagic food web (Bowman and McCain Citation1967; Yoo Il, Citation1970). This carnivorous amphipod species consumes zooplankton, therefore serving as a trophic link between smaller organisms and larger zooplankton predators (Haro-Garay Citation2003). Additionally, C. challengeri have a high essential fatty acid content, which indicates they are a nutrient-rich food source for their predators (Costalago et al. Citation2020; Hiltunen et al. Citation2022). Currently, there are no mitochondrial genomes available for the Family Cyphocarididae Lowry & Stoddart Citation2003, and no available sequences for C. challengeri. In this study, we detail the complete mitogenome of C. challengeri to provide insight into the evolutionary history and phylogenetic relationships.
Materials and methods
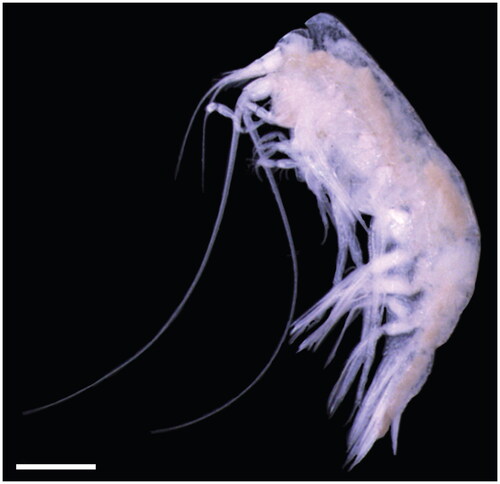
Eight C. challengeri individuals were collected from Carr Inlet, Puget Sound (47°16’35.76” N, 122°42’29.52” W) on 15 September 2019 at 17:18 UTC using a 335-μm mesh bongo net towed obliquely to 90 m depth at the 105 m deep station P38. Specimens were identified by A. Winans, a trained taxonomist in J. Keister’s laboratory at the University of Washington, comparing characteristics with Hughes and Lowry (Citation2015) taxonomic key (whole organism photographed for ). Genomic DNA was extracted from the body tissue of one whole specimen (T367) using the DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. DNA quality was checked on a 1.5% agarose gel and quantified using a Qubit 4 Fluorometer. The DNA extract is archived with the Ocean Molecular Ecology group at the NOAA Pacific Marine Environmental Laboratory (contact corresponding author) under specimen identification T367. High-throughput paired-end 2 × 150 bp sequencing was performed at Azenta Life Sciences (South Plainfield, NJ) on an Illumina HiSeq 4000 after library prep with the NEBNext Ultra DNA Library Prep Kit, producing 48.9 million raw paired reads totaling 14.7 Gbp.
Figure 1. Photograph of the Cyphocaris challengeri specimen sequenced in this study. Scale bar 1 mm. Photo taken by C. Rabinowitz.
Raw reads were trimmed to remove adaptors using Trimmomatic 0.39 (Bolger et al. Citation2014) and then merged using Flash 1.2.11 (Magoc and Salzburg, Citation2011). Adaptor-trimmed merged and remaining unmerged reads were then quality-filtered using a 4 bp Q20 sliding window in Trimmomatic. Quality filtering produced 31.4 million paired reads and 16.7 million unpaired reads (including both merged and quality filtered unpaired reads) totaling 12.6 Gbp. Quality-controlled reads were then assembled using SPAdes 3.15.5 with k-mer sizes at 21, 55, 85, 115, and 127 bp assuming an isolate genome (Prjibelski et al. Citation2020). A complete, circular mitogenome with 12.7x coverage was pulled from this assembly using GetOrganelle (Jin et al. Citation2020). Accuracy of the completed mitogenome was confirmed by aligning the quality-controlled reads (Q30 mapping score) to the final mitogenome product using Bowtie 2 (Supplemental Figure 1) (Langmead and Salzberg Citation2012). Mitogenome annotation was completed using the MITOS web server (Bernt et al. Citation2013), with additional annotation using MitoZ (Meng et al. Citation2019) to identify the trnL1 gene. Small and large subunit rRNA genes were annotated by hand using reference amphipod mitogenomes aligned to rRNA secondary structures (Pons et al. Citation2014). Visualizations of the mitogenome annotation were modified from outputs generated by Geneious Prime (v.2022), including standard percent GC calculations.
To confirm the phylogenetic placement and taxonomic assignment of C. challengeri, we aligned the amino acids of all 13 protein-encoding genes available from Amphipoda mitogenomes (111 circular and 87 linear) using MUSCLE 3.8.425 (Edgar Citation2004), and masked the alignment to remove any position containing >30% gaps. A maximum likelihood phylogenetic tree was constructed from this alignment using the PROTGAMMAJTT model in RAxML 8.2.12, with 300 bootstrap replicates (Stamatakis Citation2014). The resulting phylogenetic tree was plotted using Iroki (Moore et al. Citation2020). References for mitogenomes used in the phylogenetic tree are provided in Supplemental Table 1.
Results
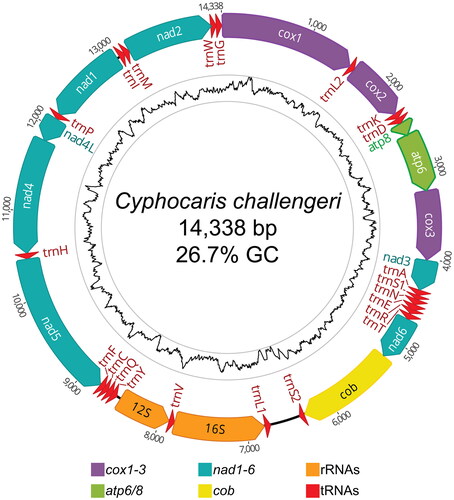
The assembled circular mitogenome of C. challengeri (accession OQ064431) is 14,338 bp long with an average GC content of 26.7% (35.5% A, 37.9% T, 18.0% C, 8.7% G). The mitogenome contains 13 protein-encoding genes, 2 ribosomal RNAs, and 22 tRNAs (). Four different start codons were found: ATG (n = 5), ATT (n = 4), TTG (n = 2), and ATA (n = 2). The control region is likely located between the trnL1 and trnS2 genes, in an AT-rich (85.8%) 339 bp non-coding region with poly-T motifs, though experimental evidence is required to confirm. The mitochondrial genome was reordered from the original assembly to begin with the start codon for cytochrome oxidase I (COI). In addition to the mitogenome, we recovered two haplotypes of the complete nuclear rRNA-ITS region (accessions OQ093270 and OQ093271), with haplotype B containing a 5 bp and 16 bp insertion in the ITS2 region compared to haplotype A (see notes on Genbank record).
Figure 2. Circular mitogenome map of Cyphocaris challengeri. All annotated genes are indicated with their direction. Inner tract shows GC content with an 80 bp sliding window; high (51% GC) and low (5% GC) values are bounded by the gray rings.
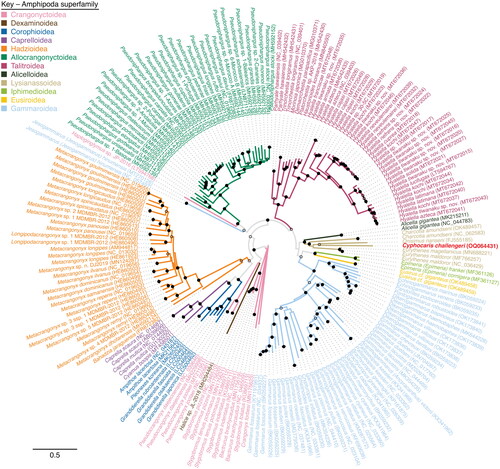
Phylogenetic analysis puts C. challengeri as a sister taxon to Onisimus nanseni (Sars Citation1900), with the next closest relatives in the genus Eurythenes (Smith Citation1882) (). As expected, the closest relatives of C. challengeri all reside within the superfamily Lysianassoidea (Dana Citation1849), forming a monophyletic clade. A similarly constructed tree based on nucleotide sequences and adding the rRNA genes showed a similar topology, with the exception of the two Jesogammarus (Jesogammarus) hinumensis (Morino Citation1993) sequences, which fall basal to the Lysianassoidea with low bootstrap support (data not shown). The protein-encoding and rRNA gene orientation and order for C. challengeri most closely resemble other amphipods from the parvorder Lysianassidira, particularly Eurythenes magellanicus (Milne Edwards Citation1848), Eurythenes maldoror (D'Udekem d'Acoz and Havermans Citation2015), and Alicella gigantea (Chevreux Citation1899). However, its closest relative, O nanseni, does not share this mitogenome order.
Figure 3. Maximum likelihood phylogenetic tree of a concatenated alignment of all thirteen protein encoding genes available from Amphipoda mitogenomes. Cyphocaris challengeri label is highlighted in red. Branches are colored by superfamily. Bootstrap values are indicated at nodes with a solid dot (≥75% support), open dot (≥50%, <75% support), or no dot (<50% support).
Discussion and conclusion
The complete mitochondrial genome and nuclear rRNA-ITS region of C. challengeri presented in this study is the first complete genomic reference data for the Cyphocarididae family. Currently, only partial-length COI sequences are registered in NCBI (Cyphocaris bouvieri (Chevreux Citation1916), Cyphocaris tunicola (Lowry and Stoddart Citation1997), and non-speciated BOLD voucher specimens). Thus, this work will enhance our ability to detect this species and its close relatives in environmental DNA (eDNA) observatories, improving ecosystem monitoring and modeling (Mathieu et al. Citation2020).
Ethical approval
No ethics approvals were required for the amphipod specimens collected in this project because the target invertebrate species is not listed as endangered, protected, or regulated. The waters of the Puget Sound do not require permits for access.
Authors’ contributions
The conception, design, and methodology of this study were completed by CNR, SDB, SMM, JEK, and MPG. The collection and identification of samples were completed by AKW and CNR. The analysis and interpretation of data were completed by SMM and MPG. All authors were involved in the drafting and revising of the paper. All authors agreed the final version to be published and agreed to be accountable for all aspects of the work.
Supplemental Material
Download TIFF Image (4 MB)Supplemental Material
Download MS Excel (17.5 KB)Acknowledgements
We thank the captain and crew of the R/V Rachel Carson. We also thank the UW Washington Ocean Acidification Center for providing the opportunity to collect these samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The complete mitochondrial genome of Cyphocaris challengeri is available on NCBI under accession number OQ064431. The complete rRNA-ITS region of two haplotypes (A/B) is available under accession number OQ093270 and OQ093271, respectively. The raw sequencing data from the C. challengeri genome is available through NCBI’s SRA, under BioProject, SRA, and BioSample accession numbers PRJNA913968, SRR22972441, and SAMN32316678, respectively.
Additional information
Funding
References
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319. doi: 10.1016/j.ympev.2012.08.023.
- Boeck A. 1871. Crustacea amphipoda borealia et arctica. Forhandlinger i Videnskabs-Selskabet i Christiana. 1870:83–280.
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30(15):2114–2120. doi: 10.1093/bioinformatics/btu170.
- Bowman TE, McCain JC. 1967. Variation and distribution of the pelagic amphipod Cyphocaris challengeri in the Northeast Pacific (Gammaridea: lysianassidae). Proceedings of the United States National Museum. 122(3588):1–14. doi: 10.5479/si.00963801.122-3588.1.
- Chevreux E. 1899. Sur deux espèces géantes d'amphipodes provenant des campagnes du yacht Princesse Alice. Bull Soc Zool France. 24:152–158. doi: 10.5962/bhl.part.24435.
- Chevreux E. 1916. Sur les Amphipodes du genre Cyphocaris Boeck recueillis par la "Princesse Alice" au moyen du filet Richard à grande ouverture. Bull Institut Océanogr Monaco. 319:1–6.
- Costalago D, Forster I, Nemcek N, Neville C, Perry RI, Young K, Hunt BPV. 2020. Seasonal and spatial dynamics of the planktonic trophic biomarkers in the Strait of Georgia (northeast Pacific) and implications for fish. Sci Rep. 10(1):8517. doi: 10.1038/s41598-020-65557-1.
- Dana JD. 1849. Synopsis of the genera of Gammaracea. Am J Sci Arts 8(ser. 2):135–140.
- D'Udekem d'Acoz C, Havermans C. 2015. Contribution to the systematics of the genus Eurythenes S.I. Smith in Scudder, 1882 (Crustacea: Amphipoda: Lysianassoidea: Eurytheneidae). Zootaxa. 3971: 1–80. doi: 10.11646/zootaxa.3971.1.1.
- Edgar RC. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 5(1):113. doi: 10.1186/1471-2105-5-113.
- Edwards MH. 1848. Sur un crustacé amphipode, remarquable par sa grande taille. Ann Sci Naturelles Zool. 9(Ser. 3):398.
- Haro-Garay MJ. 2003. Diet and functional morphology of the mandible of two planktonic amphipods from the Strait of Georgia, British Columbia, Parathemisto pacifica (Stebbing, 1888) and Cyphocaris challengeri (Stebbing, 1888). Crustac. 76(11):1291–1312. doi: 10.1163/156854003323009821.
- Hiltunen M, Strandberg U, Brett MT, Winans AK, Beauchamp DA, Kotila M, Keister JE. 2022. Taxonomic, temporal, and spatial variations in zooplankton fatty acid composition in Puget Sound, WA, USA. Estuaries Coasts. 45(2):567–581. doi: 10.1007/s12237-021-00973-8.
- Hughes LE, Lowry JK. 2015. A review of the world Cyphocarididae with description of three new species (Crustacea, Amphipoda, Lysianassoidea). Zootaxa. 4058(1):1–40. doi: 10.11646/zootaxa.4058.1.1.
- Jin JJ, Yu WB, Yang J-B, Song Y, dePamphilis CW, Yi TS, Li DZ. 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21(1):241. doi: 10.1186/s13059-020-02154-5.
- Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods. 9(4):357–359. doi: 10.1038/nmeth.1923.
- Lowry JK, Stoddart HE. 1997. Amphipoda Crustacea IV. Families Aristiidae, Cyphocarididae, Endevouridae, Lysianassidae, Scopelocheiridae, Uristidae. Ser Memoirs Hourglass Cruises. 10:148.
- Lowry JK, Stoddart HE. 2003. Crustacea: Malacostraca: Peracarida: Amphipoda, Cumacea, Mysidacea. In: P. L. Beesly & W. W. K. Houston, editors. Zoological Catalogue of Australia, p. 92–93. Collingwood, Victoria, Australia: CSIRO Publishing.
- Magoc T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 27(21):2957–2963. doi: 10.1093/bioinformatics/btr507.
- Mathieu C, Hermans SM, Lear G, Buckley TR, Lee KC, Buckley HL. 2020. A systematic review of sources of variability and uncertainty in eDNA data for environmental monitoring. Front Ecol Evol. 8:135. doi: 10.3389/fevo.2020.00135.
- Meng G, Li Y, Yang C, Liu S. 2019. MitoZ: a toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 47(11):e63–e63. doi: 10.1093/nar/gkz173.
- Morino H. 1993. A new species of the genus Jesogammarus (Amphipoda: Anisogammaridae) from brackish waters of Japan. Publication Itako Hydrobiological Station. 6:9–16.
- Moore RM, Harrison AO, McAllister SM, Polson SW, Wommack KE. 2020. Iroki: automatic customization and visualization of phylogenetic trees. PeerJ. 8:e8584. doi: 10.7717/peerj.8584.
- Pons J, Bauzà-Ribot MM, Jaume D, Juan C. 2014. Next-generation sequencing, phylogenetic signal and comparative mitogenomic analyses in Metacrangonyctidae (Amphipoda: Crustacea). BMC Genomics. 15(1):566. doi: 10.1186/1471-2164-15-566.
- Prjibelski A, Antipov D, Meleshko D, Lapidus A, Korobeynikov A. 2020. Using SPAdes de novo assembler. Curr Protoc Bioinformatics. 70(1):e102. doi: 10.1002/cpbi.102.
- Sars GO. 1900. Crustacea. Scientific Results of the Norwegian North Polar Expedition 1893–1896. 1:1–141.
- Smith SI. 1882. Nomenclator Zoologicus. An alphabetical list of all generic names that have been employed by naturalists for recent and fossil animals from the earliest times to the close of the year 1879. In 2 parts: 1. Supplemental list. II. Universal index. BullUS Natl Museum. (19). i–xxi, 1–376, 1–340. doi: 10.5479/si.03629236.19.i.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenetics. Bioinformatics. 30(9):1312–1313. doi: 10.1093/bioinformatics/btu033.
- Stebbing TRR. 1888. Report on the Amphipoda collected by H.M.S challenger during the years 1873-1876. Zoology. 29:1–212.
- WoRMS Editorial Board. 2022. World Register of Marine Species. doi: 10.14284/170.
- Yoo K Il. 1970. On the distribution of the pelagic amphipod, Cyphocaris challengeri (Gammaridea: Lysianassidae) in the western North Pacific. Korean J Zool. 13(3):94–100. https://koreascience.kr/article/JAKO197011919884679.page.
