Abstract
The authors sequenced the complete mitochondrial (mt) genomes of the band-legged ground cricket (Dianemobius fascipes nigrofasciatus Matsumura, 1904) and a temperate form of the lawn ground cricket (Polionemobius taprobanensis Walker, 1869), collected in Japan. The length of the mt genome sequences was 15,354 bp in D. fascipes nigrofasciatus and 16,063 bp in P. taprobanensis. Annotation of the mt genome sequences revealed 13 protein-coding genes, two rRNA genes, and 22 tRNA genes. The orientation of the genes was the same as in other Grylloidea species, and the order was the same as in other Trigonidiidae species. In our phylogenetic analysis, D. fascipes nigrofasciatus formed a clade with D. fascipes collected in China, and the temperate form of P. taprobanensis formed a clade with P. taprobanensis collected in China. Comparison of the numbers of positions with different amino acid residues encoded by the protein-coding genes implied the separate species status of each member of each of the two pairs of ground crickets. The mt genome sequences of D. fascipes nigrofasciatus and P. taprobanensis will contribute to phylogenetic and taxonomic studies of the Trigonidiidae.
Introduction
The band-legged ground cricket, Dianemobius fascipes nigrofasciatus (Matsumura 1904), is distributed in temperate regions such as southern Siberia, Northeast China, Korea, and Japan (Hokkaido, Honshu, Kyushu, Shikoku islands) (Benediktov and Storozhenko Citation2018). A subspecies, Dianemobius fascipes fascipes (Walker, 1869) is distributed in subtropical regions such as India, Sri Lanka, Nepal, Myanmar, Thailand, Vietnam, Malaysia, Indonesia, the Philippines, South and Southeast China, and Japan (Ryukyu Islands) (Benediktov and Storozhenko Citation2018). Taxonomically, D. fascipes nigrofasciatus has been confirmed to be a subspecies of D. fascipes fascipes (nominotypical subspecies) by differences in the acoustic calling signals, the color of the hind tibia, the shape of the ectoparamere lobe, and similarities in the male genital morphology (Benediktov and Storozhenko Citation2018). Male hybrids between temperate (= D. fascipes nigrofasciatus) and subtropical (= D. fascipes fascipes) forms are almost always sterile (Masaki Citation1983).
Contrary to the clear taxonomic status of the Dianemobius group, there has been controversy in the taxonomic status and notation of the lawn ground cricket, Polionemobius taprobanensis (Walker, 1869). As far as we know, the newest description concerning this classification was that Polionemobius mikado (Shiraki, 1911) is a synonym for a northern group of P. taprobanensis (He Citation2018). Polionemobius taprobanensis is distributed in Korea, Japan, Russia (far east), China, Taiwan, Southeast Asia, Indonesia, India, and Sri Lanka (Storozhenko et al. Citation2015). According to Masaki (Citation1983), temperate (= northern) and subtropical (= southern) forms of the lawn ground cricket in Japan, with Tokunoshima (Ryukyu Islands) as the border of their distribution, were distinguished on the basis of differences in their ovipositor length, diapause, and photoperiodic responses. Masaki (Citation1983) also argued that the names Pteronemobius mikado and Pteronemobius taprobanensis should be applied to the temperate and subtropical forms, respectively. However, Storozhenko et al. (Citation2015) found no substantial differences in the shape of the male genitalia among specimens from Sri Lanka, India, Southeast Asia, China, Japan, Korea, and Russia. They further showed that the ovipositor length varied among females sampled in the same area. Masaki (Citation1983) reported that hybrids of the temperate and subtropical forms and their F2 forms are fertile.
These ground crickets are often used as models of geographic variation (Masaki Citation1979a, Citation1979b, Citation1979c, Citation1983; Matsuda et al. Citation2018), diapausing (Masaki Citation1979a, Citation1983; Shiga and Numata Citation1997; Matsuda and Numata Citation2019), and photoperiodic response (Masaki Citation1979a, Citation1983). Interestingly, the distribution patterns are similar in the two pairs D. fascipes fascipes—D. fascipes nigrofasciatus and temperate—subtropical forms of P. taprobanensis (Masaki Citation1983). Furthermore, D. fascipes nigrofasciatus and the temperate form of P. taprobanensis exhibit photoperiod-dependent egg diapause, while D. fascipes fascipes and the subtropical form of P. taprobanensis have no diapause (Masaki Citation1983). The sequences of the mitochondrial (mt) genomes of D. fascipes with no subspecific names provided and P. taprobanensis of unknown form collected in China have already been registered (GenBank: MK303550.1 and NC_045848.1) (Ma et al. Citation2019b). Here, we determined the mt genome sequences of D. fascipes nigrofasciatus and a temperate form of P. taprobanensis, as well as their phylogenetic status.
Materials and methods
Adult specimens of D. fascipes nigrofasciatus and P. taprobanensis were collected from the ground at the Institute of Marine and Coastal Research of Ochanomizu University, Tateyama, Chiba, Japan (34.98°N, 139.82°E), under the permission by Prof. Masato Kiyomoto, the director of the facility. Specimens of D. fascipes nigrofasciatus () were distinguished from D. fascipes fascipes by the color patterns on the hind femurs and tibias (Benediktov and Storozhenko Citation2018). Specimens of P. taprobanensis were the temperate form distinguished from the subtropical form by the difference in ovipositor length. The length in P. taprobanensis of our specimen turned out to be 3.4 mm, as measured using NIH ImageJ (ver. 1.53k; Schneider et al. Citation2012), which corresponds to the length described in the temperate form by Masaki (Citation1983). Genomic DNA of these two species was extracted using a QIAamp DNA Mini Kit (QIAGEN, Venlo, The Netherlands) in accordance with the manufacturer’s manual. Since genomic DNA was extracted from whole bodies of the samples after the specimen identification performed by Kohyoh Murata, no body parts were retained but original photographs of the specimens are stored as e-vouchers and openly accessible on figshare at https://doi.org/10.6084/m9.figshare.21781610.v3. Furthermore, other individuals of the same two species, which were collected in the same region and at the same time as the samples used in this study, are deposited as secondary specimens in the Center for Molecular Biodiversity Research, National Museum of Nature and Science (https://www.kahaku.go.jp/; 4-1-1 Amakubo, Tsukuba, Ibaraki 305-0005, Japan; Utsugi Jinbo, [email protected]), under voucher numbers NSMT-DNA 53743 and NSMT-DNA 53744 for D. fascipes nigrofasciatus and the temperate form of P. taprobanensis, respectively. The mt genomes were sequenced using the NovaSeq 6000 platform (Illumina, San Diego, California) and assembled using GetOrganelle (ver. 1.7.3.4-pre; Jin et al. Citation2020). According to the protocol previously described (https://www.protocols.io/view/generating-sequencing-depth-and-coverage-map-for-o-4r3l27jkxg1y/v1), we validated the assembly by mapping all raw reads to the mitochondrial genomes and evaluating the sequencing coverage depth (Fig. S1). Automatic annotation was performed by MITOS and MITOS2 web servers (Bernt et al. Citation2013; Donath et al. Citation2019). To compare protein-coding gene (PCG) sequences in the Grylloidea, mt genome data of another 17 Grylloidea species were downloaded from NCBI Genome at https://www.ncbi.nlm.nih.gov/genome/. Alignment of the PCG sequences using MEGAⅩ (ver. 10.1.8; Kumar et al. Citation2018) showed evident discordances in gene start and termination points compared with those of the other cricket species. The annotation was therefore manually curated. Phylogenetic analyses were performed using MEGAⅩ and were based on the sequences of all 13 PCGs of the 19 crickets; the disturbed regions in their alignment were removed using trimAl (ver. 1.4. rev15; Capella-Gutiérrez et al. Citation2009). We generated an initial phylogenetic tree using the neighbor-joining method (Saitou and Nei Citation1987) and then generated the final tree using the maximum likelihood method (Felsenstein Citation1981). To test the significance of branching in the tree, the bootstrap method was adopted for both analyses (Felsenstein Citation1985). For the analyses using neighbor joining, the Jones-Taylor-Thornton model (Jones et al. Citation1992) with gamma distribution adjustment was adopted, and for those using the maximum likelihood method, the mtREV model (Adachi and Hasegawa Citation1996) with adjustments of frequency, invariant sites, and gamma distribution was adopted, in accordance with an advanced model test in MEGAⅩ. Sequences of 13 PCGs of the Drosophila melanogaster subgroup (Drosophila mauritiana (NC_005779), Drosophila simulans (MN046104), Drosophila sechellia (MK659840), and Drosophila melanogaster (U37541)) were downloaded from NCBI to compare the numbers of positions with different amino acid residues between pairs of these species and the two pairs of crickets.
Results
The length of the mt genome of D. fascipes nigrofasciatus was 15,354 bp, and that of the temperate form of P. taprobanensis was 16,063 bp (). These included the 13 PCGs (nad2, cox1, cox2, atp8, atp6, cox3, nad3, nad5, nad4, nad4L, nad6, cob, and nad1), two rRNA genes (rrnL and rrnS), and 22 tRNA genes (trnI, trnQ, trnM, trnW, trnC, trnY, trnL2, trnK, trnD, trnG, trnA, trnR, trnE, trnS1, trnN, trnF, trnH, trnT, trnP, trnS2, trnL1, and trnV), of which the orientation was the same as in other Grylloidea species (Kataoka et al. Citation2020; Sanno et al. Citation2021). The gene order around trnV is rrnL–rrnS–trnV in Trigonidiidae species, although it is rrnL–trnV–rrnS in many other Grylloidea species (Ma et al. Citation2019b; Sanno et al. Citation2021). Gene order characteristic of the Trigonidiidae was observed in D. fascipes nigrofasciatus and the temperate form of P. taprobanensis. Non-canonical start codons were observed in cox1 and nad1 of D. fascipes nigrofasciatus and in cox1, nad1, and nad3 of the temperate form of P. taprobanensis. Previous studies have shown the presence of non-canonical start codons in cox1 and nad1 in Orthopteran insects (Sheffield et al. Citation2010; Sanno et al. Citation2021), but none of the previous reports had found a TTG start codon in nad3. We checked the read coverage depth of this region (6206–6208 nt) and found that the TTG start codon in nad3 was likely genuine (). Our phylogenetic analysis confirmed that D. fascipes nigrofasciatus and P. taprobanensis belonged to the Trigonidiidae family (). In addition to the Trigonidiidae, our phylogenetic tree generated three other clades—Gryllidae, Phalangopsidae, and Mogoplistidae—of which the topology corresponded to that in previous studies (; Ma et al. Citation2019b; Sanno et al. Citation2021). In addition, D. fascipes nigrofasciatus and the temperate form of P. taprobanensis formed clades with D. fascipes with no subspecific names provided and P. taprobanensis of unknown form collected in China, respectively. The branch length from the divergence of D. fascipes collected in China and D. fascipes nigrofasciatus was almost the same as that of P. taprobanensis collected in China and the temperate form (). We found that the number of positions with different amino acid types encoded by the PCGs between D. fascipes collected in China and D. fascipes nigrofasciatus was 53, and that between P. taprobanensis collected in China and the temperate form was 49; these values were in the range of the differences between pairs of the four sister species of the D. melanogaster subgroup ().
Figure 2. Maps of the mitochondrial genomes of Dianemobius fascipes nigrofasciatus and Polionemobius taprobanensis (temperate form). the outer and inner rings represent heavy and light chains, respectively. Different colors indicate different gene families. The darker and lighter gray area in the inner circle represent the GC and AT contents, respectively. Reads mapped at the TTG start codon (red frame, 6206–6208 nt) in nad3 of the P. taprobanensis mitochondrial genome were also shown in right.
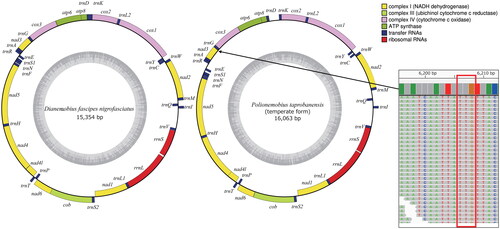
Figure 3. Maximum likelihood phylogenetic tree of 19 Grylloidea species with 1000 bootstraps. Bold text denotes species of which the sequences were newly revealed in this study. The amino acid sequences of the 13 PCGs of following species were also used: L. equestris KU562919 (Yang et al. Citation2016), L. doenitzi NC_033985 (unpublished), V. hemelytrus NC_030762 (Yang et al. Citation2016), X. marmoratus NC_041236 (Ma et al. Citation2019a), C. muiri NC_037914 (Dong et al. Citation2017), T. hibinonis NC_034797 (Li et al. Citation2019), O. sinensis NC_034799 (Li et al. Citation2019), M. japonica NC_039665 (Ma and Li Citation2018), C. rogenhoferi NC_039664 (Ma and Li Citation2018), T. sjostedti NC_032077 (Song et al. Citation2016), H. nigripes NC_045841 (Ma et al. Citation2019b), P. taprobanensis (unknown form) NC_045848 (Ma et al. Citation2019b), P. taprobanensis (temperate form) OP854597, D. furumagiensis NC_045847 (Ma et al. Citation2019b), D. fascipes nigrofasciatus OP854596, D. fascipes (unknown form) NC_045846 (Ma et al. Citation2019b), O. kanetataki NC_039667 (Ma and Li Citation2018), O. fuscicercis NC_039739 (Ma and Li Citation2018), and O. bimaculatus NC_039666 (Ma and Li Citation2018).
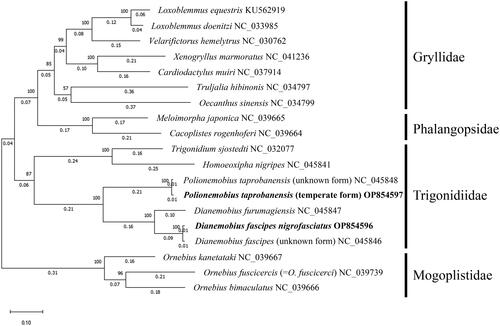
Table 1. Numbers of amino acid differences in the 13 protein-coding genes.
Discussion
These findings suggest that, within each pair, D. fascipes collected in China—D. fascipes nigrofasciatus collected in Japan and P. taprobanensis collected in China—the temperate form collected in Japan, are closely related, but different species. Each pair can be more easily determined by the cox1 sequence; the former pair differed by 12 nt in the nucleotides from 1006 to 1368 and the latter pair differed by 7 nt in the nucleotides from 1204 to 1436. It is unclear whether the two crickets collected in China (Ma et al. Citation2019b) correspond to D. fascipes fascipes and the subtropical form of P. taprobanensis, respectively. In Japan, the members of each pair of D. fascipes fascipes—D. fascipes nigrofasciatus and temperate—subtropical forms of P. taprobanensis diverged by allopatric speciation, because their distributions are divided at about 25°N to 28°N in the Ryukyu Islands (Masaki Citation1983). Further studies, such as phylogenetic analyses by using mt genomes including the subtropical species, whole genomes, or population genetics, are required to reveal the details of these speciations.
Conclusion
In summary, we revealed the complete mt genomes of two Trigonidiidae crickets, D. fascipes nigrofasciatus and the temperate form of P. taprobanensis collected in Japan, and found by phylogenetic analysis that they were closely related to D. fascipes and P. taprobanensis collected in China, respectively. The PCGs in the mt genomes of the two pairs of crickets seemed to encode proteins with enough amino acid differences for each member of each pair to be considered as a different species. These divergences are likely examples of allopatric speciation because of the division in the species’ distribution areas. Crickets have been used as models for studying speciation (Kataoka et al. Citation2022). The Trigonidiidae mt genome sequences revealed here will contribute to phylogenetic and taxonomic studies of crickets to elucidate the mechanisms of speciation.
Ethical approval
This research does not involve ethical research. Insects are invertebrates, and there are no ethics involved in using them in experiments.
Author contributions
KM, KK, AO, TA, KY, and TS conceived and planned the study. KM, KK, and RS performed sample collection, wet processes, and bioinformatics analyses. KS supported the bioinformatics analyses. All the authors wrote the manuscript.
Supplemental Material
Download PDF (1.1 MB)Acknowledgments
The authors thank Prof. Masato Kiyomoto (Tateyama Marine Laboratory, Institute of Marine and Coastal Research, Ochanomizu University) for his support in sampling the crickets used in this study. We also thank the hosts of the website orthoptera.jp at https://www.orthoptera-jp.com/ for sharing the literature on cricket classification.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/under the accession nos. OP854596 (D. fascipes nigrofasciatus) and OP854597 (temperate form of P. taprobanensis). The associated BioProject, SRA, and Bio-Sample numbers are PRJNA926495 (D. fascipes nigrofasciatus and temperate form of P. taprobanensis), SRX19142015 (D. fascipes nigrofasciatus) and SRX19142016 (temperate form of P. taprobanensis), and SAMN32875128 (D. fascipes nigrofasciatus) and SAMN32875129 (temperate form of P. taprobanensis), respectively.
Additional information
Funding
References
- Adachi J, Hasegawa M. 1996. Model of amino acid substitution in proteins encoded by mitochondrial DNA. J Mol Evol. 42(4):459–468. doi: 10.1007/BF02498640.
- Benediktov AA, Storozhenko SY. 2018. Confirmation of the subspecies status of Dianemobius fascipes nigrofasciatus (Orthoptera, Gryllidae) based on bioacoustic and morphological data, with the description of the male sounds from Southern Siberia. Entmol Rev. 98(8):1038–1044. doi: 10.1134/S0013873818080109.
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319. doi: 10.1016/j.ympev.2012.08.023.
- Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 25(15):1972–1973. doi: 10.1093/bioinformatics/btp348.
- Donath A, Jühling F, Al-Arab M, Bernhart SH, Reinhardt F, Stadler PF, Middendorf M, Bernt M. 2019. Improved annotation of protein-coding genes boundaries in metazoan mitochondrial genomes. Nucleic Acids Res. 47(20):10543–10552. doi: 10.1093/nar/gkz833.
- Dong J, Vicente N, Chintauan-Marquier IC, Ramadi C, Dettai A, Robillard T. 2017. Complete mitochondrial genome and taxonomic revision of Cardiodactylus muiri Otte, 2007 (Gryllidae: eneopterinae: lebinthini). Zootaxa. 4268(1):101–116. doi: 10.11646/zootaxa.4268.1.6.
- Felsenstein J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 17(6):368–376. doi: 10.1007/BF01734359.
- Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 39(4):783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x.
- He ZQ. 2018. A checklist of Chinese crickets (Orthoptera: gryllidea). Zootaxa. 4369(4):515–535. doi: 10.11646/zootaxa.4369.4.4.
- Jin J-J, Yu W-B, Yang J-B, Song Y, dePamphilis CW, Yi T-S, Li D-Z. 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21(1):241. doi: 10.1186/s13059-020-02154-5.
- Jones DT, Taylor WR, Thornton JM. 1992. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 8(3):275–282. doi: 10.1093/bioinformatics/8.3.275.
- Kataoka K, Minei R, Ide K, Ogura A, Takeyama H, Takeda M, Suzuki T, Yura K, Asahi T. 2020. The draft genome dataset of the Asian cricket teleogryllus occipitalis for molecular research toward entomophagy. Front Genet. 11:470. doi: 10.3389/fgene.2020.00470.
- Kataoka K, Togawa Y, Sanno R, Asahi T, Yura K. 2022. Dissecting cricket genomes for the advancement of entomology and entomophagy. Biophys Rev. 14(1):75–97. doi: 10.1007/s12551-021-00924-4.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35(6):1547–1549. doi: 10.1093/molbev/msy096.
- Li J, Chen Q, Wen M, Wang J, Wang Y, Ren B. 2019. Phylogeny and acoustic signal evolution of a pure tone song katydid Pseudophyllus titan (Orthoptera: tettigoniidae) based on the complete mitogenome. Mitochondrial DNA A DNA Mapp Seq Anal. 30(3):385–396. doi: 10.1080/24701394.2018.1502280.
- Ma C, Li J. 2018. Comparative analysis of mitochondrial genomes of the superfamily Grylloidea (Insecta, Orthoptera) reveals phylogenetic distribution of gene rearrangements. Int J Biol Macromol. 120(Pt A):1048–1054. doi: 10.1016/j.ijbiomac.2018.08.181.
- Ma C, Wang Y, Zhang L, Li J. 2019b. Mitochondrial genome characterization of the family Trigonidiidae (Orthoptera) reveals novel structural features and nad1 transcript ends. Sci Rep. 9(1):19092. doi: 10.1038/s41598-019-55740-4.
- Ma C, Zhang L, Li J. 2019a. Characterization of the complete mitochondrial genome of a bush cricket Xenogryllus marmoratus (Insecta: Orthoptera). Mitochondrial DNA Part B: resources. 4(1):172–173. doi: 10.1080/23802359.2018.1544870.
- Masaki S. 1979a. Climatic adaptation and species status in the lawn ground cricket: I. Photoperiodic response. 47(l):48–65. https://hirosaki.repo.nii.ac.jp/?action=pages_view_main&active_action=repository_view_main_item_detail&item_id=4391&item_no=1&page_id=13&block_id=33.
- Masaki S. 1979b. Climatic adaptation and species status in the lawn ground cricket: II. Body size. Oecologia. 35(3):343–356. doi: 10.1007/BF00345141.
- Masaki S. 1979c. Climatic adaptation and species status in the lawn ground cricket: III. Ovipositor length. Oecologia. 43(2):207–219. http://www.jstor.org/stable/4215955. doi: 10.1007/BF00344771.
- Masaki S. 1983. Climatic speciation in Japanese ground crickets. GeoJournal. 7(6):483–490. http://www.jstor.org/stable/41143194. doi: 10.1007/BF00218520.
- Matsuda N, Numata H. 2019. Altitudinal variation in life-history traits in the lawn ground cricket, Polionemobius mikado. Entomol Sci. 22(2):198–204. doi: 10.1111/ens.12359.
- Matsuda N, Tanaka K, Watari Y, Shintani Y, Goto SG, Nisimura T, Izumi Y, Numata H. 2018. Northward expansion of the bivoltine life cycle of the cricket over the last four decades. Glob Chang Biol. 24(12):5622–5628. doi: 10.1111/gcb.14436.
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454.
- Sanno R, Kataoka K, Hayakawa S, Ide K, Nguyen CN, Nguyen TP, Le BTN, Kim OTP, Mineta K, Takeyama H, et al. 2021. Comparative analysis of mitochondrial genomes in Gryllidea (Insecta: orthoptera): implications for adaptive evolution in ant-loving crickets. Genome Biol Evol. 13(10):1–8. doi: 10.1093/gbe/evab222.
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Method. 9(7):671–675. doi: 10.1038/nmeth.2089.
- Sheffield NC, Hiatt KD, Valentine MC, Song H, Whiting MF. 2010. Mitochondrial genomics in orthoptera using MOSAS. Mitochondrial DNA. 21(3–4):87–104. doi: 10.3109/19401736.2010.500812.
- Shiga S, Numata H. 1997. Seasonal changes in the incidence of embryonic diapause in the band-legged ground cricket, Dianemobius nigrofasciatus. Zoological Sci. 14(6):1015–1018. doi: 10.2108/zsj.14.1015.
- Song N, Li H, Song F, Cai W. 2016. Molecular phylogeny of Polyneoptera (Insecta) inferred from expanded mitogenomic data. Sci Rep. 6(1):36175. doi: 10.1038/srep36175.
- Storozhenko SY, Kim T-W, Jeon MJ. 2015. Monograph of Korean Orthoptera. National Institute of Biological Resources: Incheon, Korea. https://www.researchgate.net/publication/316662596_Monograph_of_Korean_Orthoptera.
- Yang J, Ren Q, Huang Y. 2016. Complete mitochondrial genomes of three crickets (Orthoptera: Gryllidae) and comparative analyses within Ensifera mitogenomes. Zootaxa. 4092(4):529–547. doi: 10.11646/zootaxa.4092.4.4.

