Abstract
Zygophyllum brachypterum Karelin & Kirilov belongs to Zygophyllaceae and is mainly distributed in the desert regions of Central Asia, Mongolia, and Northwest China. The species is valuable in exploring the adaptations of Zygophyllaceae plants to salt stress in ecological environments. In this study, we report the complete chloroplast (cp) genome of Z. brachypterum. The entire cp genome was 104590 bp in length, with a large single-copy region (LSC, 79170 bp), a small single-copy region (SSC, 16778 bp), and two inverted repeats (IRa/IRb) of 4321 bp each. A total of 106 genes were detected, among which seven were located in the IRs, and 65, 30, and 4 were protein-coding, tRNA, and rRNA genes, respectively. Notably, eleven genes encoding the subunits of NAD(P)H dehydrogenase complex (NDH) were absent. Phylogenetic analysis indicated that Z. brachypterum belonged to Zygophylloideae (Zygophyllaceae). Furthermore, it was closely related to Z. fabago and Z. kansuense.
Introduction
Zygophyllum brachypterum Karelin & Kirilov 1841 belongs to Zygophylloideae within Zygophyllaceae, according to the current Angiosperm Phylogeny Group IV classification. It is a perennial herb that is primarily found in the arid and semi-arid desert areas and river valleys of Central Asia, Mongolia, and the Xinjiang Autonomous Region of China (). Zygophyllaceae is xerophytic with strong drought adaptation and resistance to wind erosion. It is also an essential ecological resource for maintaining the fragile ecosystem of its habitats (Wu et al. Citation2018). Some Zygophyllum species are strongly resistant to salt and alkali environments and can survive in barren and quicksand areas (Yang and Furukawa Citation2006), as well as in the presence of some heavy metals in soil (Parraga-Aguado et al. Citation2013; Lefèvre et al. Citation2014). Z. brachypterum harbors such characteristics, and a previous study has explored its strategies for coping with salt stress (Wang et al. Citation2020). Recently, there has been little focus on the other characteristics of Z. brachypterum, and its complete cp genome has not yet been reported. In this study, the complete cp genome of Z. brachypterum was assembled and annotated to analyze its structural characteristics. A Maximum Likelihood (ML) phylogenetic tree was constructed based on the common protein-coding genes (PCGs) of 14 species from Krameriaceae and Zygophyllaceae to confirm the phylogenetic status of the related species.
Figure 1. Specimen of Zygophyllum brachypterum (this unpublished photo, taken in Aksu Prefecture, Xinjiang Uygur Autonomous Region of China by Prof. Peipei Jiao, is used with permission), Stems of Z. brachypterum are much branched and tender, and leaves have two leaflets that are oblong to oblanceolate, thin, and apex obtuse. Petioles are equal to or shorter than leaflets.

Materials and methods
The seeds of Z. brachypterum were collected from Baicheng County, Aksu Prefecture, Xinjiang Uygur Autonomous Region of China (N41°50.425′, E82°01.130′). The specimen was stored at the Herbarium, Kunming Institute of Botany (http://www.genobank.org/, Huajie He, [email protected]) under the voucher number KUN 1246197. The total genome was isolated from fresh leaves cultured in the laboratory using the Plant Genomic DNA Kit (DP305, Tiangen Biotech Co., China) and sequenced on the Illumina HiSeq 2500 platform. The entire cp genome was assembled using Getorganelle v1.7.7.0 and annotated using GeSeq (https://chlorobox.mpimp-golm.mpg.de/geseq.html). The primary reads and complete Z. brachypterum cp genome sequence were deposited to GenBank (reads: SRR24097901, PRJNA952865, SAMN34090572; cp genome: accession number NC _081907). The genes in each region were generated using OrganellarGenomeDRAW (OGDRAW) v. 1.3.1. A phylogenetic tree based on 53 common PCGs was constructed using 14 relevant species from Krameriaceae, Balanitoideae, Larreoideae, Tetraena and Zygophyllum belonging to Zygophylloideae of Zygophyllaceae, with Amborella trichopoda as the outgroup. Nucleotide sequences were retrieved from the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/). The PCGs were filtered using PhyloSuite v1.2.3 (Zhang et al. Citation2020; Xiang et al. Citation2023), and multiple sequence alignments were performed using MAFFT (Katoh et al. Citation2019). Results were optimized using MACS v2 (Ranwez et al. Citation2018) and concatenated using PhyloSuite v1.2.3 (Zhang et al. Citation2020; Xiang et al. Citation2023). Evolutionary analyses were conducted in MEGA 11 (Tamura et al. Citation2021) using the ML method with 1000 bootstrap replications and the GTR + G + I model.
Results
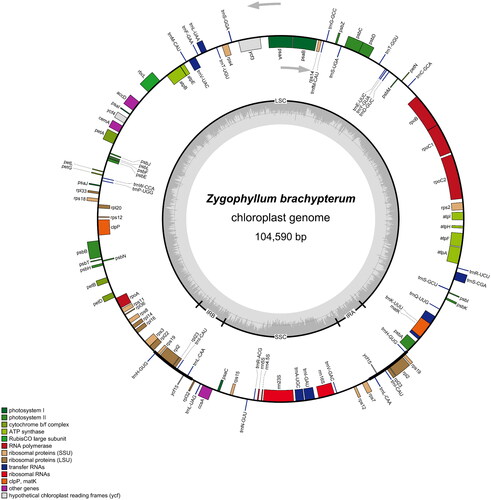
The sequencing depth and coverage map for Z. brachypterum cp genome assembly was shown in Figure S1 (supplementary material). The PCR gel image of Z. brachypterum cp genome was displayed in Figure S2 (supplementary material). The complete Z. brachypterum cp genome was 104590 bp in length, with a typical quadripartite structure in a circular form and an overall ratio of GC content of 33.94%. Two large inverted repeats (IRa/IRb) of 4321 bp-long each divided the entire genome into a large single-copy (LSC; 79170 bp) region and a small single-copy (SSC; 16778 bp) region. The total number of genes was 106, of which seven (trnL-CAA, ycf15, trnI-CAU, rpl23, rpl2, rps19, and trnH-GUG) were located in the IR region. The 99 unique genes consisted of 65 protein-coding and 30 tRNA genes. Notably, only four rRNA genes were located in the SSC region, unlike most other angiosperms. Furthermore, eleven ndh genes encoding the subunits of NAD(P)H dehydrogenase complex (NDH) were not detected in the cp genome. Additionally, infA, rps16, ycf1, and ycf2 were absent. Eleven genes contained introns (ycf3 with two introns; and trnK-UUU, trnS-CGA, trnL-UAA, trnV-UAC, trnA-UGC, trnI-GAU, atpF, rpoC1, rpl2, and clpP with one intron each). Notably, clpP had two introns in the cp genome of species belonging to other subfamilies from Zygophyllaceae (Yan et al. Citation2019; Gonçalves et al. Citation2019a; Al-Juhani et al. Citation2022). The cis-splicing genes were trnI-GAU, rpl2, trnS-CGA, rpoC1, trnV-UAC, ycf3, clpP, trnA-UGC, trnL-UAA, atpF and trnK-UUU in Z. brachypterum cp genome, and the trans-splicing gene rps12 had only two exons, which were located in the LSC and SSC, respectively. The schematic maps of the cis-splicing genes and trans-splicing gene in Z. brachypterum cp genome were shown in Figure S3 (supplementary material). Moreover, no introns were found in rpl16, petB, and petD in the Z. brachypterum cp genome (). All of the above features may be attributed to the short sequence length of the Z. brachypterum cp genome compared to other cp genomes of angiosperms. No apparent rearrangements or inversions were observed in the Z. brachypterum cp genome.
Figure 2. The complete chloroplast genome map of Z. brachypterum. The quadripartite structure is marked with LSC, SSC, and IRA/IRB. Different functional genes are represented by bars with different colors. The gray part in the inner circle indicates the GC content. Genes outside the circle are transcribed in the clockwise direction, and those inside the circle are transcribed in the counterclockwise direction.
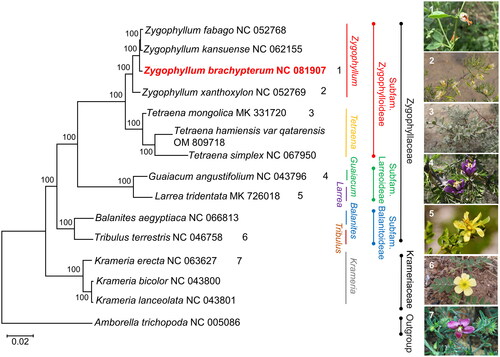
To confirm the evolutionary status of Z. brachypterum, a phylogenetic analysis was conducted to construct an ML tree based on the PCGs contained in all fourteen related species from Krameriaceae and Zygophyllaceae, using A. trichopoda as the outgroup (). Results showed that Z. brachypterum belonged to Zygophyllum in the subfamily Zygophylloideae from Zygophyllaceae, which is consistent with the conclusion of APG IV. Z. brachypterum was the sister species of the branch consisting of Z. fabago and Z. kansuense, and this group together with Z. xanthoxylum belonged to Zygophyllum. The branch of Zygophyllum and the three Tetraena species clustered into the subfamily Zygophylloideae, which was separated from Zygophyllaceae following the generation of Balanitoideae and Larreoideae.
Figure 3. The phylogenetic tree is based on the common PCGs of 14 related species from Krameriaceae and Zygophyllaceae. A. trichopoda is regarded as the outgroup. The bootstrap values based on 1000 replications are exhibited at the branches. Scale bar = 0.02. The images of Z. brachypterum, Z. xanthoxylum, and T. mongolica were taken from Polat Muhtar and Ningmei Chen with permission for use. The images of Guaiacum angustifolium, Larrea tridentata, Tribulus terrestris, and Krameria erecta were downloaded from the public domain and can be used without asking permission as declared by the copyright holders. The following sequences downloaded from NCBI were used: Zygophyllum fabago NC_052768 (Xu et al. Citation2020), Zygophyllum kansuense NC_062155 (Wang et al. Citation2022a), Zygophyllum xanthoxylum NC_052769 (Xu et al. Citation2020), Tetraena mongolica MK331720 (Wang et al. Citation2022b), Tetraena hamiensis var. qatarensis OM809718 (Ahmad et al. Citation2023), Tetraena simplex NC_067950 (Ahmad et al. Citation2023), Guaiacum angustifolium NC_043796 (Gonçalves et al. Citation2019b), Larrea tridentata MK726018 (Gonçalves et al. Citation2019a), Balanites aegyptiaca NC_066813 (Al-Juhani et al. Citation2022), Tribulus terrestris NC_046758 (Yan et al. Citation2019), Krameria erecta NC_063627 (Banerjee et al. Citation2022), Krameria bicolor NC_043800 (Gonçalves et al. Citation2019b), Krameria lanceolata NC_043801 (Gonçalves et al. Citation2019b), and Amborella trichopoda NC_005086 (Goremykin et al. Citation2003).
Discussion and conclusion
The entire set of eleven ndh genes was absent in the Z. brachypterum cp genome, which was consistent with the reports for T. mongolica, Z. xanthoxylum, and Z. fabago (Wang et al. Citation2022b). Loss of plastid ndh genes was observed in Pinaceae/Gnetales (Wakasugi et al. Citation1994) and monocotyledon lineage orchids (Wu et al. Citation2010). Recently, eleven ndh genes appeared as pseudogenes that occurred in the long branch formed in thirteen Erodium species (Blazier et al. Citation2011). Whether the loss of ndh genes in the cp genome is a typical evolutionary characteristic of Zygophylloideae requires more information. Likewise, plastid genomes of other species in this subfamily should be further explored.
This first report of the complete Z. brachypterum cp genome is a valuable addition to the plastid genome data of Zygophyllum species. This clarifies the evolutionary position of Z. brachypterum and provides a basis for subsequent studies on the phylogeny of Zygophylloideae and Zygophyllaceae, and other characteristics of Z. brachypterum and Zygophyllum species, aside from resistance strategies and adaptation to the environment.
Ethical approval
This study includes no human, animal, or endangered plant samples, and the material involved in this study does not involve ethical conflicts. All collection and laboratory works were conducted under local legislation and related laboratory regulations.
Author contributions
Li W and Zhou YJ conceived and designed this work. Wang XY performed the experiments and was responsible for the analysis and interpretation of the data. The manuscript was written by Wang XY. Zhou YJ and Gao F reviewed the intellectual content. All authors approved the final version to be published and agreed to be accountable for all aspects of the work.
Supplemental Material
Download TIFF Image (1.1 MB)Supplemental Material
Download TIFF Image (6.6 MB)Supplemental Material
Download TIFF Image (2.2 MB)Supplemental Material
Download TIFF Image (2.6 MB)Acknowledgments
The authors sincerely thank Prof. Peipei Jiao (Tarim University), Assoc. Prof. Polat Muhtar (Xinjiang University) and Lect. Ningmei Chen (Guizhou University of Traditional Chinese Medicine) for providing the plant images in the field.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under the accession number NC_081907. The associated BioProject, SRA, and BioSample numbers are PRJNA952865, SRR24097901, and SAMN34090572, respectively.
Additional information
Funding
References
- Ahmad W, Asaf S, Al-Rawahi A, Al-Harrasi A, Khan AL. 2023. Comparative plastome genomics, taxonomic delimitation and evolutionary divergences of Tetraena hamiensis var. qatarensis and Tetraena simplex (Zygophyllaceae). Sci Rep. 13(1):7436. doi: 10.1038/s41598-023-34477-1.
- Al-Juhani WS, Alharbi SA, Al Aboud NM, Aljohani AY. 2022. Complete chloroplast genome of the desert date (Balanites aegyptiaca (L.) Del. comparative analysis, and phylogenetic relationships among the members of Zygophyllaceae. BMC Genomics. 23(1):626. doi: 10.1186/s12864-022-08850-9.
- Banerjee A, Schneider AC, Stefanovi S. 2022. Plastid genomes of the hemiparasitic genus Krameria (Zygophyllales) are intact and exhibit little relaxation in selection. Int J Plant Sci. 183(5):393–403. doi: 10.1086/719959.
- Blazier JC, Guisinger MM, Jansen RK. 2011. Recent loss of plastid-encoded ndh genes within Erodium (Geraniaceae). Plant Mol Biol. 76(3–5):263–272. doi: 10.1007/s11103-011-9753-5.
- Gonçalves DJP, Simpson BB, Ortiz EM, Shimizu GH, Jansen RK. 2019a. Incongruence between gene trees and species trees and phylogenetic signal variation in plastid genes. Mol Phylogenet Evol. 138:219–232. doi: 10.1016/j.ympev.2019.05.022.
- Gonçalves DJP, Simpson BB, Shimizu GH, Jansen RK, Ortiz EM. 2019b. Genome assembly and phylogenomic data analyses using plastid data: contrasting species tree estimation methods. Data Brief. 25:104271. doi: 10.1016/j.dib.2019.104271.
- Goremykin VV, Hirsch-Ernst KI, Wolfl S, Hellwig FH. 2003. Analysis of the Amborella trichopoda chloroplast genome sequence suggests that amborella is not a basal angiosperm. Mol Biol Evol. 20(9):1499–1505. doi: 10.1093/molbev/msg159.
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 20(4):1160–1166. doi: 10.1093/bib/bbx108.
- Lefèvre I, Vogel-Mikuš K, Jeromel L, Vavpetič P, Planchon S, Arčon I, Van Elteren JT, Lepoint G, Gobert S, Renaut J, et al. 2014. Differential cadmium and zinc distribution in relation to their physiological impact in the leaves of the accumulating Zygophyllum fabago L. Plant Cell Environ. 37(6):1299–1320. doi: 10.1111/pce.12234.
- Parraga-Aguado I, Gonzalez-Alcaraz MN, Alvarez-Rogel J, Jimenez-Carceles FJ, Conesa HM. 2013. The importance of edaphic niches and pioneer plant species succession for the phytomanagement of mine tailings. Environ Pollut. 176:134–143. doi: 10.1016/j.envpol.2013.01.023.
- Ranwez V, Douzery EJP, Cambon C, Chantret N, Delsuc F. 2018. MACSE v2: toolkit for the alignment of coding sequences accounting for frameshifts and stop codons. Mol Biol Evol. 35(10):2582–2584. doi: 10.1093/molbev/msy159.
- Tamura K, Stecher G, Kumar S. 2021. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 38(7):3022–3027. doi: 10.1093/molbev/msab120.
- Wakasugi T, Tsudzuki J, Ito S, Nakashima K, Tsudzuki T, Sugiura M. 1994. Loss of all ndh genes as determined by sequencing the entire chloroplast genome of the black pine Pinus thunbergii. Proc Natl Acad Sci USA. 91(21):9794–9798. doi: 10.1073/pnas.91.21.9794.
- Wang J, Jiang X, Zhao CF, Fang ZM, Jiao PP. 2020. Transcriptomic and metabolomic analysis reveals the role of CoA in the salt tolerance of Zygophyllum spp. BMC Plant Biol. 20(1):9. doi: 10.1186/s12870-019-2226-8.
- Wang N, Wang S, Zhang L, Chang ZY. 2022a. The complete chloroplast genome sequence of Zygophyllum kansuense Y. X. Liou (Zygophyllaceae). Mitochondrial DNA B Resour. 7(10):1864–1866. doi: 10.1080/23802359.2022.2135396.
- Wang XY, Dorjee T, Chen YR, Gao F, Zhou YJ. 2022b. The complete chloroplast genome sequencing analysis revealed an unusual IRs reduction in three species of subfamily Zygophylloideae. PLoS One. 17(2):e0263253. doi: 10.1371/journal.pone.0263253.
- Wu FH, Chan MT, Liao DC, Hsu CT, Lee YW, Daniell H, Duvall MR, Lin CS. 2010. Complete chloroplast genome of Oncidium Gower Ramsey and evaluation of molecular markers for identification and breeding in Oncidiinae. BMC Plant Biol. 10(1):68. doi: 10.1186/1471-2229-10-68.
- Wu SD, Zhang LJ, Lin L, Yu SX, Chen ZD, Wang W. 2018. Insights into the historical assembly of global dryland floras: the diversification of Zygophyllaceae. BMC Evol Biol. 18(1):166. doi: 10.1186/s12862-018-1277-z.
- Xiang CY, Gao FL, Jakovlić I, Lei HP, Hu Y, Zhang H, Zou H, Wang GT, Zhang D. 2023. Using PhyloSuite for molecular phylogeny and tree‐based analyses. Imeta. 2(1):e87. doi: 10.1002/imt2.87.
- Xu HM, Fu WR, Xie W, Wang YG, Zhang YF. 2020. The complete chloroplast genomes of two species of Zygophyllum (Zygophyllaceae). Mitochondrial DNA B Resour. 5(3):3476–3477. doi: 10.1080/23802359.2020.1825132.
- Yan JK, Zhang N, Duan Y. 2019. The complete chloroplast genome sequence of Tribulus terrestris, an important traditional Chinese medicine. Mitochondrial DNA B Resour. 4(2):3108–3109. doi: 10.1080/23802359.2019.1667891.
- Yang SM, Furukawa I. 2006. Anatomical adaptations of three species of Chinese xerophytes (Zygophyllaceae). J of for Res. 17(3):247–251. doi: 10.1007/s11676-006-0056-7.
- Zhang D, Gao FL, Jakovlić I, Zou H, Zhang J, Li WX, Wang GT. 2020. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour. 20(1):348–355. doi: 10.1111/1755-0998.13096.
