Abstract
The family Hippoboscidae is an ectoparasite that primarily inhabits bats and relies on the host’s blood for sustenance. This research provides the first complete mitochondrial genome of Nycteribia formosana, which shares similar characteristics with other dipteran insects. The circularized mitochondrial genome, spanning 15,107 bp, encompasses 13 protein-coding genes (PCGs), 22 transfer RNA genes (tRNA), two ribosomal RNA genes, and a control region. The nucleotide composition of A, C, G, and T is 40.4%, 10.9%, 6.7%, and 42.0%, respectively. The findings from the phylogenetic analysis suggest that the species under investigation forms a cluster with other species belonging to the family Nycteribiidae. Consequently, this study provides valuable insights for the identification of N. formosana.
Introduction
The family Nycteribiidae, which falls under the order Diptera and the superfamily Hippoboscoidea, consists of highly specialized blood-feeding flies that exclusively associate with bats (Mammalia: Chiroptera). These flies, known as bat flies along with the family Streblidae, are obligate body surface parasites of bats. They parasitize the host’s fur and wing membranes, primarily feeding on the host’s blood (Dick and Dittmar Citation2013). Nycteribiids exhibit distinct morphological features: this group is characterized by their complete lack of wing and spider-like appearance, the thorax is dorsoventrally flattened, head size is reduced, and eyes are either reduced or completely absent (Dick and Patterson Citation2006).
Bats are widely believed to serve as the primary source of transmission for numerous zoonotic pathogens. Among these pathogens, bat flies have been identified as potential carriers and significant contributors to the transmission and persistence of bat borne diseases. In recent decades, a substantial number of pathogens have been detected in bats and their ectoparasites on a global scale (Han et al. Citation2021), including Bartonella (McKee et al. Citation2021), haemosporidian (Sándor et al. Citation2021), Rickettsia, Mahlapitsi virus (Szentiványi et al. Citation2019), and others. The presence of these pathogens poses a serious threat to human safety.
Based on the available data, Nycteribia formosana (Karaman, 1939) is predominantly a parasite of the genus Myotis and was initially detected in Taiwan, China (Orlova et al. Citation2021). Other documented occurrences of this species have been reported in Korea and Russia (Mogi et al. Citation2023). Notably, N. formosana shares striking morphological similarities with Nycteribia uenoi, thereby posing difficulties in their taxonomic differentiation (Kim et al. Citation2012). Consequently, the accurate identification of N. formosana presents a considerable challenge. Thus, in light of these findings, our study has contributed to the comprehensive characterization of the mitochondrial genome of N. formosana, enhanced the knowledge regarding species within the Nycteribiidae family, and established a solid genomic resource base for future investigations.
Materials
Sample collection
N. formosana samples were obtained from Myotis fimbriatus individuals captured in Shibao Mountain (26°24′13″N, 99°50′31″E) in Jianchuan County, Dali City, Yunnan Province, China. These samples were subsequently identified by Xiaoyan Zheng through microscopic examination of their morphological characteristics. Following identification, individual N. formosana specimens were carefully prepared and preserved at the Pathogen and Vector Biology Research Institute of Dali University (website: https://www.dali.edu.cn/kxyj/yjs/1611.htm; contact person: Xiaobin Huang, [email protected]) under the voucher number DLBC2022001. The samples were stored at 95% ethanol and −20 °C.
Statement
The capture, procedures, and handling of Myotis fimbriatus were conducted in accordance with the regulations and guidelines established by the Ethics Committee of Dali University, under the approval number MECDU-202104-27. This study adhered rigorously to ethical standards and guidelines pertaining to animal handling.
Methods
DNA extraction, mitogenome sequencing, and annotation
First, the genomic DNA of N. formosana was extracted using the Tissue DNA Kit (Omega, Norcross, GA). The experiment utilized a single individual to complete the genome assembly. The complete mitochondrial genome of N. formosana was sequenced using the Illumina Novoseq 6000 sequencing platform. The type of flowcell used for Illumina is MiSeq flowcell, which has a read length of 150,150 and is a paired read. In this particular study, the MitoZ 2.3 software (https://doi.org/10.1093/nar/gkz173) was employed for the assembly of the mitochondrial genome (Meng et al. Citation2019), while MITOS (Bernt et al. Citation2013) was utilized for annotation purposes. Lastly, a circular mitochondrial genome map was generated using the online platform GenomeVx (Conant and Wolfe Citation2008). The nucleotide sequence of N. formosana has been deposited in the GenBank database under the accession number OQ675011.
Sequence annalysis
The nucleotide composition of N. formosana was analyzed using Geneious Prime software (Kearse et al. Citation2012). Chironomus tepperi (Beckenbach Citation2012) and Dixella aestivalis (Briscoe et al. Citation2015) were utilized as outgroups in the construction of the phylogenetic tree. The phylogenetic tree, emcompassing N. formosana and 14 other species previously published on NCBI, was constructed using the IQ-TREE (Trifinopoulos et al. Citation2016) network server, based on the nucleotide sequences of 13 protein-coding genes (PCGs). The best-fit models for maximum-likelihood (ML) were determined through the utilization of Modelfinder (Kalyaanamoorthy et al. Citation2017). The ML phylogenetic tree was constructed employing 1000 ultrafast bootstraps under the GTR + F + I + G4 model, while support for evolutionary branches was assessed by means of a non-parametric bootstrap method with 1000 replicates. The GenBank accession numbers corresponding to the 14 species in question are provided: Basilia ansifera MZ826150 (Porter et al. Citation2022), Dipseliopoda setosa MZ826151 (Porter et al. Citation2022), Glossina austeni MZ826152 (Porter et al. Citation2022), Glossina brevipalpis MZ826153 (Porter et al. Citation2022), Lipoptena sp. MT679542 (Wang et al. Citation2021), Melophagus ovinus KX870852 (Liu et al. Citation2017), Ornithomya biloba MZ379837 (Li et al. Citation2022), Paradyschiria parvula MK896865 (Trevisan et al. Citation2019), Paratrichobius longicrus MK896866 (Trevisan et al. Citation2019), Brachytarsina amboinensis OQ301749 (Poon et al. Citation2023), Raymondia sp. OQ301747 (Poon et al. Citation2023), Phthiridium sp. OQ301748 (Poon et al. Citation2023), Phthiridium szechuanum OP459298 (Zhang et al. Citation2023), and Nycteribia parvula OP442519 (Yang et al. Citation2023).
Results and discussion
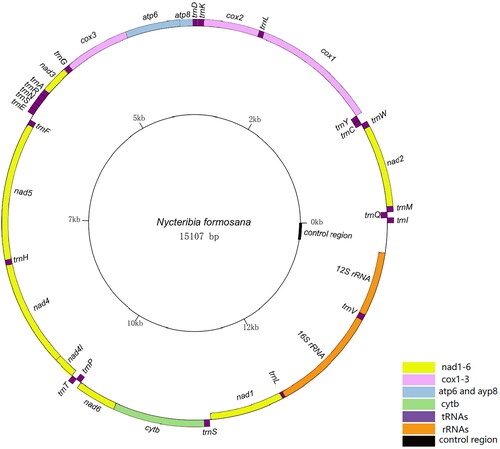
General characteristics of the mitochondrial genome
The circular mitochondrial genome of N. formosana was found to have a total length of 15,107 bp (GenBank accession number: OQ675011). This genome comprises 37 mitochondrial genes, including the standard 13 PCGs, 22 tRNA genes, two rRNA genes, and a control region. The sequence data obtained from this genome aligns with earlier investigations conducted on Diptera genomes (Porter et al. Citation2022). In the 15,107 bp nucleotide sequence of N. formosana, the distribution of nucleotides is as follows: A = 6097 (40.4%), C = 1649 (10.9%), G = 1012 (6.7%), and T = 6349 (42.0%). The combined content of A + T is notably high at 82.4%, significantly surpassing the content of G + C (17.6%). Among the 13 PCGs analyzed, it was observed that five PCGs (nad2, atp8, nad3, nad4L, nad6) commence with the initiation codon ATT, while another set of five PCGs (cox2, atp6, cox3, nad4, cytb) initiate with the codon ATG. On the other hand, cox1, nad5, and nad1 initiate with the codons TCG, TTG, and TTG, respectively. Furthermore, seven PCGs (nad2, cox2, atp8, atp6, cox3, nad4L, nad6) terminate with the codon TAA, while 3 PCGs (nad1, cytb, nad3) terminate with the codon TAG. Interestingly, cox1, nad5, and nad4 exhibit an incomplete stop codon, terminating with a single T residue, the remaining 22 transfer RNA (tRNA) sequences and two ribosomal RNA (rRNA). Sequences exhibit varying lengths, ranging from 62 bp to 1365 bp ().
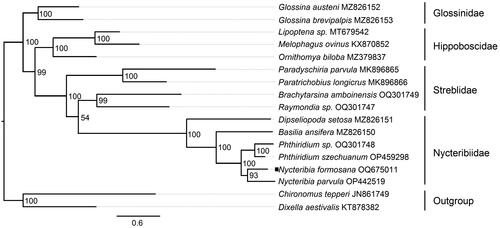
Phylogenetic analysis
According to the phylogenetic tree depicted in . formosana, Dipseliopoda setosa, Basilia ansifera, Phthiridium szechuanum, and Nycteribia parvula form a single clade for the family Nycteribiidae. The molecular phylogeny supports the placement of N. formosana in the family Nycteribiidae, and the close proximity of Nycteribia parvula provides evidence for the genus identification ().
Discussion and conclusions
This study presents a comprehensive analysis of the gene sequence of N. formosana, marking the first investigation for this species. The complete mitochondrial genome information of this species was successfully obtained. Furthermore, the phylogenetic analysis provided additional evidence supporting the homology between N. formosana and other related species. Consequently, this study effectively addresses the present limited amount of molecular data on N. formosana, thereby furnishing crucial DNA molecular information for prospective research endeavors encompassing phylogenetic studies, evolutionary analysis, and species identification within the bat flies.
Author contributions
The study was conducted by Xiaoyan Zheng, Xiaobin Huang, and Jinting Yang, with Xiaoyan Zheng being responsible for the experiment and manuscript writing. Jinting Yang and Huijuan Yang performed data analysis, while Yujuan Wang and Xianzheng Zhang primarily contributed to the construction of the phylogenetic tree. Xiaobin Huang provided critical revisions to the intellectual content. All authors have reviewed and approved the manuscript for publication and are prepared to assume accountability for all aspects of the research.
Supplemental Material
Download JPEG Image (145 KB)Supplemental Material
Download PDF (336.3 KB)Disclosure statement
All authors declare that there is no conflict of interest between them.
Data availability statement
The genomic sequence data supporting this study can be accessed from GenBank on the NCBI website (https://www.ncbi.nlm.nih.gov/) with reference number OQ675011. The associated SRA, BioSample, and BioProject numbers are SRR23951134, SAMN33858843, and PRJNA947516, respectively.
Additional information
Funding
References
- Beckenbach AT. 2012. Mitochondrial genome sequences of Nematocera (lower Diptera): evidence of rearrangement following a complete genome duplication in a winter crane fly. Genome Biol Evol. 4(2):89–101. doi:10.1093/gbe/evr131.
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319. doi:10.1016/j.ympev.2012.08.023.
- Briscoe AG, Sivell D, Harbach RE. 2015. The complete mitochondrial genome of Dixella aestivalis (Diptera: Nematocera: Dixidae). Mitochondrial DNA A DNA Mapp Seq Anal. 28(1):83–84. doi:10.3109/19401736.2015.1110809.
- Conant GC, Wolfe KH. 2008. GenomeVx: simple web-based creation of editable circular chromosome maps. Bioinformatics. 24(6):861–862. doi:10.1093/bioinformatics/btm598.
- Dick CW, Dittmar K. 2013. Parasitic bat flies (Diptera: Streblidae and Nycteribiidae): host specificity and potential as vectors. Berlin, Heidelberg: Springer Berlin Heidelberg; p. 131–155.
- Dick CW, Patterson BD. 2006. Bat flies: obligate ectoparasites of bats. Tokyo: Springer Japan; p. 179–194.
- Han H-J, Li Z-M, Li X, Liu J-X, Peng Q-M, Wang R, Gu X-L, Jiang Y, Zhou C-M, Li D, et al. 2021. Bats and their ectoparasites (Nycteribiidae and Spinturnicidae) carry diverse novel Bartonella genotypes, China. Transbound Emerg Dis. 69(4):e845–e858. doi:10.1111/tbed.14357.
- Kim HC, Han SH, Dick CW, Choi YG, Chong ST, Klein TA, Rueda LM. 2012. Geographical distribution of bat flies (Diptera: Nycteribiidae and Streblidae), including two new records, Nycteribia allotopa and N. formosana, collected from bats (Chiroptera: Rhinolophidae and Vespertilionidae) in the Republic of Korea. J Vector Ecol. 37(2):333–337. doi:10.1111/j.1948-7134.2012.00235.x.
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 14(6):587–589. doi:10.1038/nmeth.4285.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649. doi:10.1093/bioinformatics/bts199.
- Li X, Wang L, Yang D. 2022. The complete mitochondrial genome of Ornithomya biloba (Diptera, Hippoboscidae). Mitochondrial DNA B Resour. 7(5):856–858. doi:10.1080/23802359.2022.2075286.
- Liu Z-Q, Kuermanali N, Li Z, Chen S-J, Wang Y-Z, Tao H, Chen C-F. 2017. The complete mitochondrial genome of the parasitic sheep ked Melophagus ovinus (Diptera: Hippoboscidae). Mitochondrial DNA B Resour. 2(2):432–434. doi:10.1080/23802359.2017.1347832.
- McKee CD, Bai Y, Webb CT, Kosoy MY. 2021. Bats are key hosts in the radiation of mammal-associated Bartonella bacteria. Infect Genet Evol. 89:104719. doi:10.1016/j.meegid.2021.104719.
- Meng G, Li Y, Yang C, Liu S. 2019. MitoZ: a toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 47(11):e63. doi:10.1093/nar/gkz173.
- Mogi M, Kim H-C, Klein TA. 2023. Notes on Diptera Pupipara of the eastern Palaearctic region 1. The taxonomic status of species and subspecies of Hippoboscidae and Nycteribiidae described from temperate Japan and the Republic of Korea. Med Entomol Zool. 74(1):1–5. doi:10.7601/mez.74.1.
- Orlova MV, Klimov PB, Moskvitina NS, Orlov OL, Zhigalin AV, Smirnov DG, Dzhamirzoyev HS, Vekhnik VP, Pavlov AV, Emelyanova AA, et al. 2021. New records of bat flies (Diptera: Nycteribiidae), with an updated checklist of the nycteribiids of Russia. Zootaxa. 4927(3):410–430. doi:10.11646/zootaxa.4927.3.5.
- Poon ESK, Chen G, Tsang HY, Shek CT, Tsui WC, Zhao H, Guénard B, Sin SYW. 2023. Species richness of bat flies and their associations with host bats in a subtropical East Asian Region. Parasit Vectors. 16(1):37. doi:10.1186/s13071-023-05663-x.
- Porter ML, Lutz H, Steck M, Chong RA. 2022. The complete mitochondrial genomes and phylogenetic analysis of two Nycteribiidae bat flies (Diptera: Hippoboscoidea). Mitochondrial DNA B Resour. 7(8):1486–1488. doi:10.1080/23802359.2022.2107450.
- Sándor AD, Péter Á, Corduneanu A, Barti L, Csősz I, Kalmár Z, Hornok S, Kontschán J, Mihalca AD. 2021. Wide distribution and diversity of malaria-related haemosporidian parasites (Polychromophilus spp.) in bats and their ectoparasites in Eastern Europe. Microorganisms. 9(2):230. doi:10.3390/microorganisms9020230.
- Szentiványi T, Christe P, Glaizot O. 2019. Bat flies and their microparasites: current knowledge and distribution. Front Vet Sci. 6:115. doi:10.3389/fvets.2019.00115.
- Trevisan B, Alcantara DMC, Machado DJ, Marques FPL, Lahr DJG. 2019. Genome skimming is a low-cost and robust strategy to assemble complete mitochondrial genomes from ethanol preserved specimens in biodiversity studies. PeerJ. 7:e7543. doi:10.7717/peerj.7543.
- Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 44(W1):W232–W235. doi:10.1093/nar/gkw256.
- Wang M, Wang J, Guo Y, Zheng Q, Nouhoum D, Meng F. 2021. Complete mitochondrial genome of a potential vector louse fly, Lipoptena grahami (Diptera, Hippoboscidae). Mitochondrial DNA B Resour. 6(6):1752–1753. doi:10.1080/23802359.2021.1931508.
- Yang J, Huang X, Yang H, Wang Y, Zhang X, Zheng X. 2023. The complete mitochondrial genome of Nycteribia parvula Speiser, 1901 (Diptera, Nycteribiidae). Mitochondrial DNA B Resour. 8(2):276–280. doi:10.1080/23802359.2023.2169573.
- Zhang X, Huang X, Wang Y, Yang H, Yang J, Zheng X. 2023. The complete mitochondrial genome of Phthiridium szechuanum (Nycteribiidae, Diptera). Mitochondrial DNA B Resour. 8(2):211–214. doi:10.1080/23802359.2023.2171245.