Abstract
Hordeum marinum ssp. marinum (Huds.) R. J. Soreng (2003) is a halophyte wild relative of barley and wheat, which exhibits remarkable salt tolerance characteristics. In this study, we presented the first characterization of the complete chloroplast genome of H. marinum ssp. marinum. Our findings reveal that the chloroplast genome of H. marinum ssp. marinum consists of a small single-copy region (SSC: 12,715 bp), a large single-copy region (LSC: 81,130 bp), and a pair of inverted repeat regions (IRs: 21,517 bp), amounting to a total length of 136,989 bp. The chloroplast genome encodes 139 genes, including 91 protein-coding genes, 38 tRNA genes, and ten rRNA genes. By utilizing phylogenetic analysis, we determine the evolutionary position of H. marinum in Triticeae. Our study provides valuable insights into the chloroplast genome of H. marinum ssp. marinum, which may have important implications for the improvement of cereal crops.
Introduction
Hordeum marinum, known for its exceptional salt tolerance ability, is a crucial genetic resource for improving cereal crops (Huang et al. Citation2018). H. marinum is considered one of the most important wild relative species of wheat and barley in terms of providing a gene pool for enhancing the salt tolerance. Consisting of two subspecies, marinum and gussoneanum, H. marinum possesses unique characteristics of the Xa genome and is widespread in Mediterranean countries and Asia (Jakob et al. Citation2007). There is minimal difference in morphology between these two subspecies. Although the nuclear genome of H. marinum was recently published by Kuang et al. (Citation2022), the complete characterization of its chloroplast genome has not been undertaken thus far. In addition, the evolutionary relationship of H. marinum in genus Hordeum is still not well understood. Therefore, our study aims to enhance the existing scientific knowledge of the Hordeum genus by analyzing the complete chloroplast genome sequence of H. marinum ssp. marinum and determining its phylogenetic position.
Materials and methods
Plant sampling
The seeds of H. marinum ssp. marinum were obtained from NordGen, Nordic Genetic Resource Center, Sweden (latitude 10°45′8.1″, longitude 59°54′49.89″). We cultivated H. marinum in our laboratory, as shown in . Leaves from seedlings at the two-leaves and one-heart stages were employed to extract total genomic DNA using Plant Genomic DNA Kit (Tiangen Biotech, Beijing, China). The specimen was deposited in the wheat biotechnology laboratory of the Beijing Agro-Biotechnology Research Center (http://babrc.ac.cn, contact person: Suping Yu, [email protected]) under the voucher number BTBU20220515.
Sequencing, assembly, and annotation
Library preparation and genome sequencing were performed on the HiSeq X Ten platform (Novogene Biotech, Beijing, China). The quality control of the sequencing raw reads were performed using FastQC v0.12.1 (Andrews Citation2010), and low-quality reads were trimmed using Trimmomatic v0.40 (Bolger et al. Citation2014). The complete chloroplast genome was assembled using GetOrganelle v1.1.7 (Jin et al. Citation2020) and annotated by GeSeq (https://chlorobox.mpimp-golm.mpg.de/geseq.html) (Tillich et al. Citation2017). The chloroplast genome structures were visualized using Chloroplot (Zheng et al. Citation2020).
Phylogeny analysis
To further explore the phylogenetic relationship of H. marinum in Triticeae, we used the complete genome sequences of 12 species of Triticeae plants. A multiple-sequence alignment was performed using MAFFT (Katoh et al. Citation2002). Maximum-likelihood (ML) evolutionary trees were then conducted using RAxML-NG (Kozlov et al. Citation2019) with 1000 bootstrap replicates based on the chloroplast genomes of 12 species.
Results
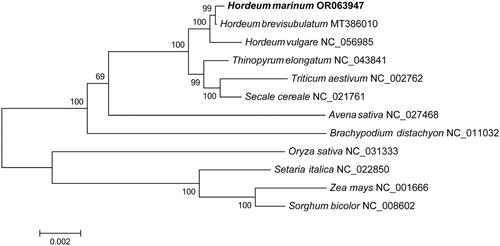
The chloroplast genome of H. marinum ssp. marinum was 136,989 bp in length. It consisted of a small single-copy region (SSC: 12,715 bp), a large single-copy region (LSC: 81,130 bp), and a pair of inverted repeat regions (IRs: 21,517 bp) that separated the SSC and LSC regions (). The average coverage depth of the chloroplast genome assembly of H. marinum ssp. marinum was 985× (Figure S1). The overall GC content of the chloroplast genome was 38.26%. A total of 139 genes were encoded in the chloroplast, including 91 protein-coding genes, 38 tRNA genes and 10 rRNA genes. Of these genes, a total of 19 contained introns, including pafI and rps12, which each had two introns. Gene structure analysis was performed for atpF, ndhA, ndhB, pafI, petB, petD, rpl16 and rps12, which were identified as difficult to annotate (Figure S2). The phylogenetic tree of 12 chloroplast genome sequences is in general consistent with genomic clustering results (Kuang et al. Citation2022), showing H. marinum and H. vulgare converged into one branch. Moreover, the phylogeny analysis revealed that H. marinum is most closely related to Hordeum brevisubulatum (), indicating their convergent evolution in response to stress.
Figure 2. Circular genetic map of the chloroplast genome of H. marinum ssp. marinum. The map consists of three circles: the inner circle indicates the positions of the LSC, SSC, IRA, and IRB regions, respectively. The Middle circle represents the GC content. The outer circle displays genes of various functional categories, each depicted in different colors as illustrated in the lower left corner of the picture.
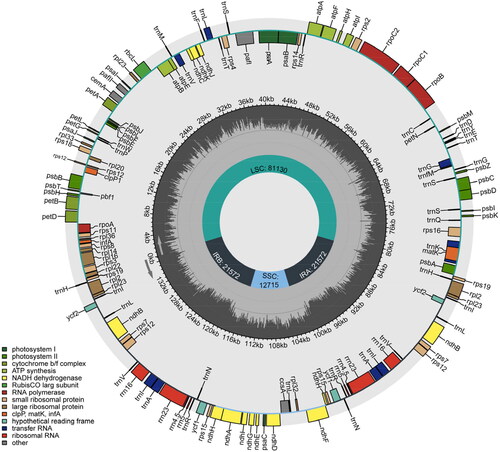
Figure 3. Phylogenetic tree inferred by ML method based on the complete chloroplast genome of 12 species. The complete chloroplast genomes of 11 species, including Hordeum brevisubulatum (MT386010) (Su et al. Citation2020), Hordeum vulgare (NC_056985), thinopyrum elongatum (NC_043841), Triticum aestivum (NC_002762) (Ogihara et al. Citation2002), secale cereale (NC_021761) (Middleton et al. Citation2014), avena sativa (NC_027468) (Saarela et al. Citation2015), brachypodium distachyon (NC_011032) (Bortiri et al. Citation2008), Oryza sativa (NC_031333), setaria italica (NC_022850), Zea mays (NC_001666) (Maier et al. Citation1995) and Sorghum bicolor (NC_008602) (Saski et al. Citation2007) were downloaded from NCBI (www.ncbi.nlm.nih.gov/).
Discussion and conclusion
Cultivating crops that are both resistant and adaptable to stress is crucial for meeting future food demands. The wild relatives of these crops possess extensive genetic diversity and exhibit strong stress resistance, making them an invaluable resource in this regard. Recently, H. marinum has gained prominence due to its unique evolutionary position and remarkable stress resistance. However, the evolutionary status of H. marinum is still not well understood due to the lack of genome sequences in Hordeum species. This study supplements and improves upon the genomics evolution analysis results of H. marinum (Kuang et al. Citation2022). It reveals that salt-tolerant H. marinum is genetically more closely related to alkali-tolerant Hordeum brevisubulatum. The finding suggests that saline alkali stress may have driven the convergent evolution of H. marinum and Hordeum brevisubulatum. Therefore, the complete chloroplast of H. marinum ssp. marinum could serve as a vital genetic resource for conducting taxonomic and evolutionary studies.
Authors’ contributions
Qingwei Du designed the research, conducted the field collection, and revised the manuscript. Suping Yu and Qingwei Du carried out the experiments, performed the data analysis, and drafted the manuscript. All authors have read and approved the final manuscript.
Ethics statement
This article does not contain any studies with human participants or animals performed by any authors. The species described in this paper are not endangered, protected, or privately owned. All plant-related procedures in this study followed the plant use guidelines of Beijing Technology and Business University and Beijing Academy of Agriculture and Forestry Sciences.
Supplemental Material
Download MS Word (4.2 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The chloroplast genome sequence data supporting the findings of this study are publicly available in NCBI. The complete chloroplast genome of H. marinum ssp. marinum was submitted to GenBank with the accession number OR063947. The associated BioProject is PRJNA976964; the SRA number is SRR24757234; and the Bio-Sample number is SAMN35447610, respectively.
Additional information
Funding
References
- Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data.
- Bolger AM, Marc L, Bjoern U. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30(15):2114–2120. doi:10.1093/bioinformatics/btu170.
- Bortiri E, Coleman-Derr D, Lazo GR, Anderson OD, Gu YQ. 2008. The complete chloroplast genome sequence of Brachypodium distachyon: sequence comparison and phylogenetic analysis of eight grass plastomes. BMC Res Notes. 1:61. doi:10.1186/1756-0500-1-61.
- Huang L, Kuang L, Li X, Wu L, Wu D, Zhang G. 2018. Metabolomic and transcriptomic analyses reveal the reasons why Hordeum marinum has higher salt tolerance than Hordeum vulgare. Environ Exp Bot. 156:48–61. doi:10.1016/j.envexpbot.2018.08.019.
- Jakob SS, Ihlow A, Blattner FR. 2007. Combined ecological niche modelling and molecular phylogeography revealed the evolutionary history of Hordeum marinum (Poaceae)-niche differentiation, loss of genetic diversity, and speciation in Mediterranean Quaternary refugia. Mol Ecol. 16(8):1713–1727. doi:10.1111/j.1365-294X.2007.03228.x.
- Jin J, Yu W, Yang J, Song Y, DePamphilis CW, Yi T, Li D. 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21(1):241. doi:10.1186/s13059-020-02154-5.
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30(14):3059–3066. doi:10.1093/nar/gkf436.
- Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A. 2019. RAxML-NG: a fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics. 35(21):4453–4455. doi:10.1093/bioinformatics/btz305.
- Kuang L, Shen Q, Chen L, Ye L, Yan T, Chen ZH, Waugh R, Li Q, Huang L, Cai S, et al. 2022. The genome and gene editing system of sea barleygrass provide a novel platform for cereal domestication and stress tolerance studies. Plant Commun. 3(5):100333. doi:10.1016/j.xplc.2022.100333.
- Maier RM, Neckermann K, Igloi GL, Kössel H. 1995. Complete sequence of the maize chloroplast genome: gene content, hotspots of divergence and fine tuning of genetic information by transcript editing. J Mol Biol. 251(5):614–628. doi:10.1006/jmbi.1995.0460.
- Middleton CP, Senerchia N, Stein N, Akhunov ED, Keller B, Wicker T, Kilian B. 2014. Sequencing of chloroplast genomes from wheat, barley, rye and their relatives provides a detailed insight into the evolution of the Triticeae tribe. PLoS One. 9(3):e85761. doi:10.1371/journal.pone.0085761.
- Ogihara Y, Isono K, Kojima T, Endo A, Hanaoka M, Shiina T, Terachi T, Utsugi S, Murata M, Mori N, et al. 2002. Structural features of a wheat plastome as revealed by complete sequencing of chloroplast DNA. Mol Genet Genomics. 266(5):740–746. doi:10.1007/s00438-001-0606-9.
- Saarela JM, Wysocki WP, Barrett CF, Soreng RJ, Davis JI, Clark LG, Kelchner SA, Pires JC, Edger PP, Mayfield DR, et al. 2015. Plastid phylogenomics of the cool-season grass subfamily: clarification of relationships among early-diverging tribes. AoB Plants. 7:plv046. doi:10.1093/aobpla/plv046.
- Saski C, Lee SB, Fjellheim S, Guda C, Jansen RK, Luo H, Tomkins J, Rognli OA, Daniell H, Clarke JL. 2007. Complete chloroplast genome sequences of Hordeum vulgare, Sorghum bicolor and Agrostis stolonifera, and comparative analyses with other grass genomes. Theor Appl Genet. 115(4):571–590. doi:10.1007/s00122-007-0567-4.
- Su X, Zhao J, Wang Z. 2020. The complete chloroplast genome of Hordeum brevisubulatum. Mitochondrial DNA B Resour. 5(3):2988–2989. doi:10.1080/23802359.2020.1797552.
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq - versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45(W1):W6–W11. doi:10.1093/nar/gkx391.
- Zheng S, Poczai P, Hyvönen J, Tang J, Amiryousefi A. 2020. Chloroplot: an online program for the versatile plotting of organelle genomes. Front Genet. 11:576124. doi:10.3389/fgene.2020.576124.

