Abstract
Dianella ensifolia (L.) Redouté 1802 is a plant known for its significant medicinal values. In this study, we presented its chloroplast genome. The length of the chloroplast genome was found to be 156,571 bp, with a GC content of 37.86%. It consisted of a large single-copy (LSC) of 85,318 bp and a small single-copy (SSC) of 18,307 bp, a pair of inverted repeats (IRs) of 26,473 bp each that separated the LSC and SSC regions. The chloroplast genome of D. ensifolia consisted of 114 unique genes, including 80 protein-coding genes, four rRNA genes, and 30 tRNA genes. Through phylogenetic analysis, we identified a close relationship between D. ensifolia and D. nigra. This newly sequenced chloroplast genome not only enhances our understanding of the genome of Dianella, but also provides valuable insights for the evolutionary study of the family Asphodelaceae.
Background
Dianella Lam. ex Juss. 1786, is a widely distributed genus found in tropical to temperate latitudes across Southeast Asia, Oceania, the Pacific Ocean, the Indian Ocean, Madagascar, and southeast Africa. Currently, there are 39 recognized species, with 22 of them occurring in Australia. Dianella species grows in a range of environments, such as grassland, heathland, sclerophyll forest, temperate to tropical rainforest and subalpine regions (Henderson Citation1987). The eastern coast of Australia is particularly rich in species diversity. Dianella ensifolia is widely distributed in China, mainly in Zhejiang, Guangzhou, Henan, and Hubei. Its root is commonly used for medicinal purposes and is known for its cool and sweet nature, as well as its anti-tumor, anti-inflammatory, and anti-aging effects (Nesterov et al. Citation2008; Tang, Huang, et al. Citation2017; Tang, Li, et al. Citation2017; Nhung et al. Citation2021).
Previous phylogenetic studies have confirmed the monophyly of Dianella using chloroplast and nuclear DNA markers (Szarowska et al. Citation2005; Muscat et al. Citation2019). However, the availability of complete chloroplast genomes for Dianella species has been limited. Only D. nigra (GenBank accession number: MN239902) has been published (Smissen and Scheele Citation2022), which has hindered phylogenetic studies of Dianella. In this study, we successfully assembled the complete chloroplast genome of D. ensifolia and conducted comparative genome analyses. Our main objective was to characterize the chloroplast genome of D. ensifolia and determine the phylogenetic position of Dianella.
Materials and methods
Material, DNA extraction, and genome sequencing
Leaves of D. ensifolia were collected from Shanghai Chen Shan Botanical Garden, Shanghai City, China (, 121.182501°E, 31.075935°N). Our experimental procedures, including plant material collection, adhered to institutional, national, or international guidelines. The collected sample was deposited at the herbarium of the Department of Plant Science and Technology, Shanghai Vocational College of Agriculture and Forestry (voucher number: DR001, Yan Zhang, [email protected]). Total genomic DNA was extracted using the CTAB method (Doyle and Doyle Citation1987). The DNA library for next-generation sequencing was constructed and sequenced using the MGISEQ platform (Benagen Technology Company, Wuhan, China). This resulted in approximately 10 Gb of raw data, which was then processed to remove low-quality sequences and obtain clean data.
Figure 1. Species reference map of D. ensifolia. This picture was taken by Yan Zhang from the Shanghai Chen Shan Botanical Garden, Shanghai City, China (voucher number: DR001; 121.182501°E, 31.075935°N). Core features: D. ensifolia is a poisonous plant. The rhizomatous stem is thick and cylindrical. Leaves: long and lanceolate. Inflorescence: panicle; tepals lanceolate, greenish white, yellowish to greenish purple. Berries: subglobose, dark blue.

Chloroplast genome assembly and annotation
De novo genome assembly was performed using GetOrganelle v.1.7.5 (Jin et al. Citation2020). For the chloroplast genome, the following parameters were applied: ‘-R 15 -k 21,45,65,85,105 -F embplant_pt’. Depth detection was performed using Samtools v.1.7 (Li et al. Citation2009) and bedtools v.2.28 (Quinlan and Hall Citation2010). The chloroplast genome was annotated using CPGAVAS2 (Shi et al. Citation2019) and PGA (Qu et al. Citation2019) with a reference genome (Hemerocallis citrina, GenBank accession number: OR260173). Annotation results were confirmed using GB2sequin (Tillich et al. Citation2017). Visualization of the chloroplast genome, cis- and trans-splicing genes was done using CPGView (Liu et al. Citation2023). Additionally, genomic hotspot analysis was performed using mVISTA (Frazer et al. Citation2004).
Repeat and IR boundary analysis
Simple sequence repeats (SSRs) were identified using the MISA software (Beier et al. Citation2017). SSRs included mono-, di-, tri-, tetra-, penta-, and hexa-nucleotides, with minimum numbers of 10, 6, 5, 5, 5, and 5, respectively. Furthermore, REPuter (Kurtz et al. Citation2001) was used to calculate palindromic, forward, reverse, and complementary repeats. The settings for REPuter were a minimum repeat size of 30 bp and hamming of 3. Additionally, IR boundaries were generated using IRscope (Amiryousefi et al. Citation2018) for comparison purposes.
Phylogenetic analysis
A phylogenetic analysis of the chloroplast genome of D. ensifolia was conducted in conjunction with other Asphodelaceae plants. A total of 79 common protein-coding genes (PCGs) were extracted from the genome annotation files using PhyloSuite v. 1.2.3 (Zhang et al. Citation2020). These genes were aligned using MAFFT v. 7.4 (Katoh and Standley Citation2013) and then concatenated. The concatenation matrix was used to construct a phylogenetic tree using the maximum-likelihood (ML) method implemented in IQ-TREE v. 2.1.2 (Nguyen et al. Citation2014). The best model (TVM + F + G4) was inferred using ModleFinder (Kalyaanamoorthy et al. Citation2017), and the bootstrap replicates were set to 1000. Tree visualization was performed using Figtree v. 1.4.3 (https://github.com/rambaut/figtree/releases/tag/v1.4.3).
Results
General features of the chloroplast genome
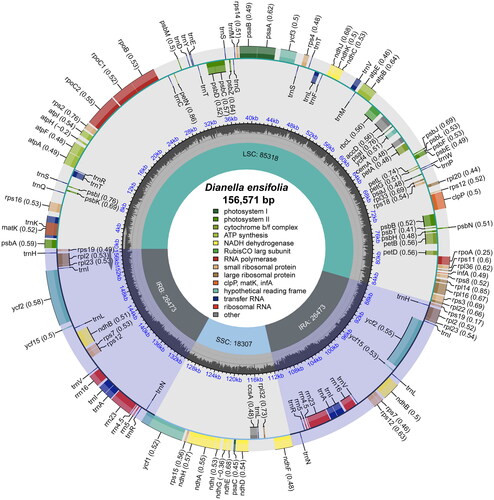
The coverage depth of the chloroplast genome and the annotation of some difficult genes were analyzed, and the results indicated a similarity between the chloroplast genome of D. ensifolia and D. nigra with some confidence (Figures S1 and S2). The chloroplast genome of D. ensifolia had a circular quadripartite structure with a length of 156,571 bp (, Table S1). It consisted of the large single-copy (LSC) region (85,318 bp), small single-copy (SSC) region (18,307 bp), and a pair of IR regions (26,473 bp). The total GC content of this chloroplast genome was 37.86%, with the GC content in the IR region (43.46%) being significantly higher than that in the LSC region (36.03%) and the SSC region (31.30%). The annotation results revealed a total of 134 genes in the chloroplast genome, with 114 unique genes remaining after removing 20 duplicated genes. These unique genes included 80 PCGs, four ribosomal RNA genes, and 30 transfer RNA genes (Tables S1 and S2). Similar to D. nigra, D. ensifolia also had eight duplicated PCGs (ndhB, rpl2, rpl23, rps7, rps12, rps19, ycf2, and ycf15), eight duplicated tRNAs (trnA-UGC, trnH-GUG, trnI-CAU, trnI-GAU, trnL-CAA, trnN-GUU, trnR-ACG, and trnV-GAC), and four duplicated rRNAs (rrn4.5, rrn5, rrn16, and rrn23). The mVISTA analysis showed that the IR region exhibited higher conservation compared to the LSC and SSC regions (Figure S3).
Figure 2. The chloroplast genome map of D. ensifolia. Genes on the inside of the circle are transcribed in a clockwise direction and genes on the outside of the circle are transcribed in a counter-clockwise direction. The optional codon usage bias is displayed in the parenthesis after the gene name. The small grey bar graphs in the inner circle show the GC contents. Genes are color-coded by their functional classification. The functional classification of the genes is shown in the center.
Repeat analysis
A total of 72 SSRs were identified in the chloroplast genome of D. ensifolia. These SSRs consisted of 67 mononucleotides, four dinucleotides, and one trinucleotide (Table S1). Mononucleotides accounted for the majority of the SSRs, making up 93.06% of the total. The SSRs were most abundant in the LSC region and least abundant in the IR region, with a higher concentration in the non-coding regions (Table S3). Additionally, 50 long repeats were identified, including 21 forward repeats, two reverse repeats, and 27 palindromic repeats (Table S1). These long repeats were mainly located in the IR region (Table S4).
IR boundaries analysis
The chloroplast genome structures of D. ensifolia and the four Hemerocallis species showed similarities, but there were minor differences in the boundaries of the inverted repeat (IR). However, the differences in IR boundaries were more pronounced when comparing Hemerocallis to D. ensifolia. Specifically, the distances between the rpl22, rps19, and trnN genes from the four boundaries (LSC-IRb, IRb-SSC, SSC-IRa, and IRa-LSC) were smaller in D. ensifolia. Furthermore, the psbA gene of D. ensifolia was located at a greater distance from the IRa-LSC boundary, the ycf1 gene was located at a greater distance within the IR region, and the ndhF gene was located at a shorter distance within the IR region.
Phylogenetic analysis
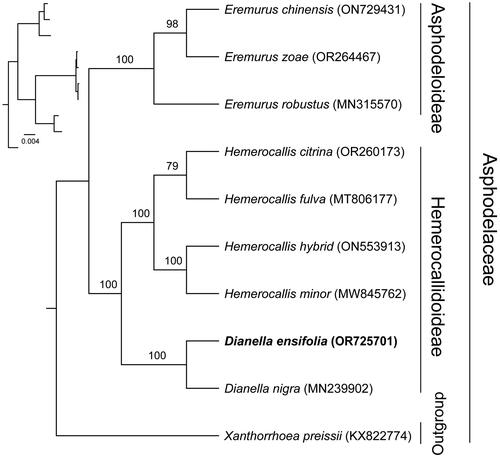
To determine the phylogenetic position of D. ensifolia, a phylogenetic analysis was conducted. The phylogenetic tree showed that our findings were generally consistent with previous studies, where most nodes have high bootstrap values (). Within Hemerocallidoideae, D. ensifolia aggregates with D. nigra as a branch, showing that they were more closely related.
Figure 3. Phylogenetic tree based on the concatenated sequences of 79 protein-coding genes in 10 species by maximum-likelihood (ML). Values split by backslashes above branches represent ML bootstraps. Dianella ensifolia (OR725701) was marked in bold. The following sequences were used: Hemerocallis hybrid (ON553913), Hemerocallis minor (MW845762) (Zhang et al. Citation2022), Hemerocallis fulva (MT806177) (Zheng et al. Citation2020), Hemerocallis citrina (OR260173) (Ou et al. Citation2020), Dianella nigra (MN239902) (Smissen and Scheele Citation2022), Eremurus chinensis (ON729431) (Makhmudjanov et al. Citation2023), Eremurus zoae (OR264467) (Makhmudjanov et al. Citation2023), Eremurus robustus (MN315570) (Makhmudjanov et al. Citation2019), Xanthorrhoea preissii (KX822774).
Discussion
Previous studies have attempted to infer the phylogenetic relationships of Dianella using partial chloroplast and nuclear DNA markers (Szarowska et al. Citation2005; Muscat et al. Citation2019). However, these studies have faced challenges in terms of low species resolution and support, which have significantly hindered phylogenetic investigations of Dianella and Asphodelaceae. To achieve accurate phylogenetic relationships, it is necessary to conduct comprehensive analyses of the nuclear and organelle genomes of a greater number of species (Górniak et al. Citation2010). Therefore, future research should focus on collecting additional Dianella material and conducting studies on its nuclear and organelle phylogenies to determine the precise phylogenetic relationships of Dianella.
Conclusions
The chloroplast genome of D. ensifolia was assembled using short-read data in this study. It showed a typical tetrameric structure, similar to the chloroplast genomes of other plants in the family Asphodelaceae. The phylogenetic tree demonstrated a clear relationship between D. ensifolia and D. nigra. Consequently, the chloroplast genome of D. ensifolia not only contributes to the genomic knowledge of Dianella but also provides insights into the evolution of the species.
Ethical approval
Leaf material of Dianella ensifolia was provided by the Shanghai Chenshan Botanical Garden. Experimental researches do not involve the genetic transformation, preservation of the genetic background of the species used, and any other processes requiring ethical approval. Therefore, no special permission was required.
Author contributions
Yan Zhang, Jian-hua Yue, and Cheng-zhong Wang conceived and designed the experiments; Jian-hua Yue and Huan Wang performed the DNA extraction experiments; Yan Zhang, Yu-bo Shi, and Xiao-ci Huang analyzed the data and modified the article; Yan Zhang and Jian-hua Yue wrote the paper. All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Excel (13.1 KB)Supplemental Material
Download MS Excel (13.7 KB)Supplemental Material
Download MS Excel (13 KB)Supplemental Material
Download MS Excel (10.8 KB)Supplemental Material
Download JPEG Image (675 KB)Supplemental Material
Download JPEG Image (4.1 MB)Supplemental Material
Download JPEG Image (429.7 KB)Supplemental Material
Download JPEG Image (138.9 KB)Supplemental Material
Download MS Word (1.4 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are available in GenBank of NCBI (http://www.ncbi.nlm.nih.gov/) under the accession no. OR725701. The associated BioProject, BioSample, and SRA numbers are PRJNA1039160, SAMN38203350, and SRR26787554, respectively.
Additional information
Funding
References
- Amiryousefi A, Hyvönen J, Poczai P. 2018. IRscope: an online program to visualize the junction sites of chloroplast genomes. Bioinformatics. 34(17):3030–3031. doi:10.1093/bioinformatics/bty220.
- Beier S, Thiel T, Münch T, Scholz U, Mascher M. 2017. MISA-web: a web server for microsatellite prediction. Bioinformatics. 33(16):2583–2585. doi:10.1093/bioinformatics/btx198.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissues. Phytochem Bull. 19:11–15.
- Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. 2004. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 32(Web Server Issue):W273–W279. doi:10.1093/nar/gkh458.
- Górniak M, Paun O, Chase MW. 2010. Phylogenetic relationships within Orchidaceae based on a low-copy nuclear coding gene, Xdh: congruence with organellar and nuclear ribosomal DNA results. Mol Phylogenet Evol. 56(2):784–795. doi:10.1016/j.ympev.2010.03.003.
- Henderson RJF. 1987. Dianella. In: George AS, editor. Flora of Australia. Vol. 45. Canberra: Australian Government Publishing Service; p. 194–225.
- Jin J-J, Yu W-B, Yang J-B, Song Y, dePamphilis CW, Yi T-S, Li D-Z. 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21(1):241. doi:10.1186/s13059-020-02154-5.
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 14(6):587–589. doi:10.1038/nmeth.4285.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780. doi:10.1093/molbev/mst010.
- Kurtz S, Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R. 2001. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 29(22):4633–4642. doi:10.1093/nar/29.22.4633.
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 25(16):2078–2079. doi:10.1093/bioinformatics/btp352.
- Liu S, Ni Y, Li J, Zhang X, Yang H, Chen H, Liu C. 2023. CPGView: a package for visualizing detailed chloroplast genome structures. Mol Ecol Resour. 23(3):694–704. doi:10.1111/1755-0998.13729.
- Makhmudjanov D, Volis S, Yusupov Z, Juramurodov I, Tojibaev K, Deng T, Sun H. 2023. Central Asia revealed as a key area in evolution of Eremurus (Asphodelaceae). Plant Divers. doi:10.1016/j.pld.2023.08.004.
- Makhmudjanov D, Yusupov Z, Abdullaev D, Deng T, Tojibaev K, Sun H. 2019. The complete chloroplast genome of Eremurus robustus (Asphodelaceae). Mitochondrial DNA B Resour. 4(2):3366–3367. doi:10.1080/23802359.2019.1674198.
- Muscat KM, Ladiges PY, Bayly MJ. 2019. Molecular phylogenetic relationships reveal taxonomic and biogeographic clades in Dianella (flax lilies; Asphodelaceae, Hemerocallidoideae). Syst Biodivers. 17(3):308–329. doi:10.1080/14772000.2019.1607617.
- Nesterov A, Zhao J, Minter D, Hertel C, Ma W, Abeysinghe P, Hong M, Jia Q. 2008. 1-(2,4-Dihydroxyphenyl)-3-(2,4-dimethoxy-3-methylphenyl)propane, a novel tyrosinase inhibitor with strong depigmenting effects. Chem Pharm Bull. 56(9):1292–1296. doi:10.1248/cpb.56.1292.
- Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. 2014. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274. doi:10.1093/molbev/msu300.
- Nhung LTH, Linh NTT, Cham BT, Thuy TT, Tam NT, Thien DD, Huong PTM, Tan VM, Tai BH, Hoang Anh NT, et al. 2021. New phenolics from Dianella ensifolia. Nat Prod Res. 35(18):3063–3070. doi:10.1080/14786419.2019.1689499.
- Ou X, Liu G, Wu L-H. 2020. The complete chloroplast genome of Hemerocallis citrina (Asphodelaceae), an ornamental and medicinal plant. Mitochondrial DNA B Resour. 5(1):1109–1110. doi:10.1080/23802359.2020.1726227.
- Qu X-J, Moore MJ, Li D-Z, Yi T-S. 2019. PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods. 15(1):50. doi:10.1186/s13007-019-0435-7.
- Quinlan AR, Hall IM. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 26(6):841–842. doi:10.1093/bioinformatics/btq033.
- Shi L, Chen H, Jiang M, Wang L, Wu X, Huang L, Liu C. 2019. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Res. 47(W1):W65–W73. doi:10.1093/nar/gkz345.
- Smissen RD, Scheele SM. 2022. Plastid genome sequences are consistent with introduction of Phormium tenax to Norfolk Island and Chatham Islands by early East Polynesian voyagers but not vicariance explanations. N Z J Bot. 60(4):429–447. doi:10.1080/0028825X.2022.2032212.
- Szarowska M, Falniowski A, Riedel F, Wilke T. 2005. Phylogenetic relationships of the subfamily Pyrgulinae (Gastropoda: Caenogastropoda: Hydrobiidae) with emphasis on the genus Dianella Gude, 1913. Zootaxa. 891(1):1–32. doi:10.11646/zootaxa.891.1.1.
- Tang B-Q, Huang S-S, Liang Y-E, Sun J-B, Ma Y, Zeng B, Lee SM-Y, Lu J-L. 2017. Two new flavans from the roots of Dianella ensifolia (L.) DC. Nat Prod Res. 31(13):1561–1565. doi:10.1080/14786419.2017.1283501.
- Tang B-Q, Li C-W, Sun J-B, Chang Y, Chan JY-W, Lee SM-Y, Zeng B. 2017. A new cycloartane-type triterpenoid from the roots of Dianella ensifolia (L.) DC. Nat Prod Res. 31(8):966–971. doi:10.1080/14786419.2016.1258558.
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq - versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45(W1):W6–W11. doi:10.1093/nar/gkx391.
- Zhang D, Gao F, Jakovlić I, Zou H, Zhang J, Li WX, Wang GT. 2020. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour. 20(1):348–355. doi:10.1111/1755-0998.13096.
- Zhang X, Lang L, Shang X, Wang Z, Jiang L, Pei X, Lu J, Li D, Yang J. 2022. The complete chloroplast genome sequence of Hemerocallis minor (Asphodelaceae). Mitochondrial DNA B Resour. 7(7):1227–1228. doi:10.1080/23802359.2022.2093663.
- Zheng Y, Li J, Chen H, Huang L. 2020. The complete chloroplast genome sequence of Hemerocallis fulva. Mitochondrial DNA B Resour. 5(3):3543–3544. doi:10.1080/23802359.2020.1829126.
