Abstract
Calystegia hederacea Wall. in Roxb. 1824 is a perennial herbaceous vine in the family Convolvulaceae and has several biological effects. Herein, we reported the first complete chloroplast genome of C. hederacea. The chloroplast genome sequence was 152,057 bp in length, comparing a large single-copy (LSC) region of 87,891 bp, a small single-copy (SSC) region of 19,866 bp, and a pair of inverted repeat (IR) regions of 22,150 bp. This sequenced chloroplast genome contained 126 predicted genes, including 81 protein-coding genes, 37 tRNA genes, and eight rRNA genes, and the total GC content of the chloroplast genome was 37.79%. Phylogenetic analysis revealed that C. hederacea was closely related to C. soldanella. The chloroplast genome presented in this study will enrich the genome information of the genus Calystegia and provide deeper insights into the evolution study of the family Convolvulaceae.
1. Introduction
The genus Calystegia R. Br. comprises approximately 26 species with wide distribution in temperate zones (Brummitt Citation1963; Brummitt and Staples Citation2007). Calystegia hederacea Wall. in Roxb. 1824, belonging to the family Convolvulaceae, is a perennial herbaceous vine with strong twining stems and large white trumpet flowers and is widely spread in India and East Asia (Hotta et al. Citation1989). Numerous studies have revealed that C. hederacea contains a series of glycosidic acids, including calyhedic acids A–F (Ono et al. Citation2020, Citation2022), calyhedins I–VI (Ono et al. Citation2021), and calysolins V–IX (Ono et al. Citation2014), and further has several biological effects, such as cytotoxicity to cancer cells, anti-bacterial, ionophoric (Pereda-Miranda et al. Citation2010), anti-viral (Ono et al. Citation2014), multidrug resistance modulatory (Figueroa-González et al. Citation2012), and anti-inflammatory (Yoshikawa et al. Citation2010). In recent years, the chloroplast genomes of several Calystegia species have been published, including C. soldanella (Wu et al. Citation2022) and C. arvensis (Wang et al. Citation2021). Nevertheless, the complete chloroplast genome of C. hederacea is still unknown. Therefore, we reported the first complete chloroplast genome of C. hederacea and further confirmed its phylogenetic position. This presented chloroplast genome will provide insight into the genomic resource for comparative genomic and species identification of the genus Calystegia.
2. Materials
The experimental samples of C. hederacea were taken from the germplasm resource nursery of Enshi Tujia and Miao Autonomous Prefecture Academy of Agricultural Sciences, Hubei Province, China (N 30°31′53″, E 109°48′11″) (). A specimen was deposited at the Herbarium of Enshi Tujia and Miao Autonomous Prefecture Academy of Agricultural Sciences (Contact: Wei Fu, [email protected]) under the voucher number XH-202309.
3. Methods
Total genomic DNA was extracted from the fresh leaves using the modified CTAB method (Doyle and Doyle Citation1987) and then randomly fragmented by sonication. Subsequently, the DNA library with an insertion size of 350 bp was constructed, and library quality was assessed on the Agilent 5400 system (Santa Clara, CA). Paired-end sequencing was performed on the DNBSEQ-T7 platform (Beijing Biomics Tech Co., Ltd., Beijing, China), yielding approximately 3.13 GB of raw data. After removing the low-quality sequences and adapter sequences by fastp (Chen et al. Citation2018), a total of 41,728,832 clean reads were obtained to de novo assemble the chloroplast genome using GetOrganelle (version 1.7.1) (Jin et al. Citation2020) with the k-mer length: 21, 35, 45, 65, 85, and 105. We further annotated the assembled chloroplast genome using CPGAVAS2 (http://47.96.249.172:16019/analyzer/annotate) (Shi et al. Citation2019) with the published chloroplast genome of C. soldanella (GenBank accession number: LC729542) (Wu et al. Citation2022) annotation as the reference. Finally, CPGView (http://47.96.249.172:16085/cpgview/view) was used to improve annotation and further identify the accuracy of cis- and trans-splicing genes (Liu et al. Citation2023).
To investigate the phylogenetic position of C. hederacea, the chloroplast genome sequence of 14 representative species was downloaded from the GenBank database to reconstruct the phylogenetic tree, with Panax ginseng (GenBank accession number: MH049735) (Wang et al. Citation2018) as an outgroup. First, PhyloSuite (version 1.2.2) (Zhang et al. Citation2020) was used to extract 69 protein-coding genes (PCGs) from the genome annotation files, then multiple sequence alignment of each gene was performed using the MAFFT (version 7.407) (Katoh and Standley Citation2013), and the aligned genes were further concatenated using PhyloSuite (version 1.2.2) (Zhang et al. Citation2020). A maximum-likelihood (ML) phylogenetic tree was constructed by IQtree (version 1.7) (Nguyen et al. Citation2015) under the best-fit model (TVM + F + I + G4) with 1000 bootstrap replicates.
4. Results
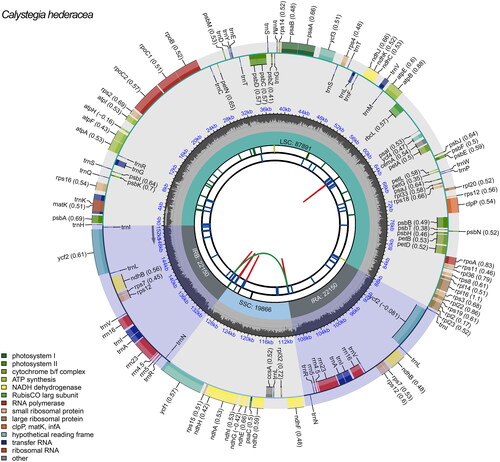
The complete chloroplast genome sequence of C. hederacea was a typical quadripartite structure with 152,057 bp in length, containing a large single-copy (LSC) region of 87,891 bp, a small single-copy (SSC) region of 19,866 bp, and a pair of inverted repeat (IR) regions of 22,150 bp (). The average coverage depth for the C. hederacea chloroplast genome is shown in Supplementary Figure 1. The overall GC content of the genome was 37.79%, whereas the corresponding value in the IR regions was 43.30%, which was higher than that in the LSC region (36.17%) and the SSC region (32.66%). This chloroplast genome contained 126 predicted genes, including 81 PCGs, 37 tRNA genes (tRNAs), and eight ribosomal RNA genes (rRNAs). Meanwhile, 10 PCGs and eight tRNAs had a single intron, and two PCGs (ycf3 and clpP) contained two introns. We also identified 12 cis-splicing genes and one trans-splicing gene in this chloroplast genome, and their structures are shown in Supplementary Figure 2.
Figure 2. Genetic map of the chloroplast genome of C. hederacea. The large single-copy (LSC), small single-copy (SSC) region, and two inverted repeat regions (IRA and IRB) are shown in the inside track. Gene models, including protein-coding, tRNA, and rRNA genes, are shown with various colored boxes in the outer track.
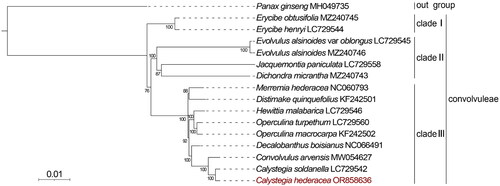
To clarify the phylogenetic position of C. hederacea, we performed a phylogenetic analysis based on 16 chloroplast genome sequences. The phylogenetic tree () showed that all the species in our study were divided into three clades, using Panax ginseng as the outgroup. Erycibe obtusifolia and Erycibe henryi were clustered in clade I. Evolvulus alsinoides var. oblongus, Evolvulus alsinoides, Jacquemontia paniculata, and Dichondra micrantha were clustered in clade II. Other species were clustered in clade III. Moreover, C. hederacea has a close relationship with C. soldanella, which was generally consistent with previous studies (Wu et al. Citation2022). These results would facilitate the phylogenetic and population genetic diversity of C. hederacea.
Figure 3. Phylogenetic relationships of C. hederacea based on the maximum-likelihood (ML) analysis of 69 protein-coding genes in chloroplast genomes. Bootstrap values next to the nodes are based on 1000 replications, and Panax ginseng was the outgroup. GenBank accession numbers: Panax ginseng MH049735 (Wang et al. Citation2018), Erycibe obtusifolia MZ240745 (Lin et al. Citation2022), Erycibe henryi LC729544 (Wu et al. Citation2022), Evolvulus alsinoides var. oblongus (Wu et al. Citation2022), Evolvulus alsinoides MZ240746 (Lin et al. Citation2022), Jacquemontia paniculata LC729558 (Wu et al. Citation2022), Dichondra micrantha MZ240743 (Lin et al. Citation2022), Merremia hederacea NC060793, Distimake quinquefolius KF242501 (Eserman et al. Citation2014), Hewittia malabarica LC729546 (Wu et al. Citation2022), Operculina turpethum LC729560 (Wu et al. Citation2022), Operculina macrocarpa KF242502 (Eserman et al. Citation2014), Decalobanthus boisianus NC 06649, Calystegia soldanella LC729542 (Wu et al. Citation2022), and Convolvulus arvensis MW054627 (Wang et al. Citation2021).
5. Discussion and conclusions
In this study, the complete chloroplast genome of C. hederacea was first sequenced and found to exhibit a typical quadripartite structure with 152,057 bp in length. Moreover, a total of 126 genes were predicted, including 81 protein-encoding genes, 37 tRNAs, and eight rRNA genes. These features are similar to those of the homologous species C. soldanella (Wu et al. Citation2022) and C. arvensis (Wang et al. Citation2021). The phylogenetic tree analysis revealed that C. hederacea was closely related to C. soldanella, which provides new data on the phylogeny, species identification, species resource conservation, and genetic investigations of C. hederacea. In the future, increasing the number of chloroplast genomes in the species of Calystegia will provide deeper insights into the evolution of this important family.
Author contributions
Yingchun Zou (Y.Z.) and Lan Long (L.L.) conceived and supervised the research. Wei Fu (W.F.), Lin Li (L.L.), and Xiaolong Wen (X.W.) conducted the bioinformatics analysis. Feifei Chen (F.C.) and Shuang Li (S.L.) contributed reagents/materials and performed the experiments. Wei Fu (W.F.) drafted the paper. Lin Li (L.L.) revised the paper critically for intellectual content. All of the authors have read and approved the final manuscript and have agreed to be accountable for all aspects of the work.
Ethical approval
No ethical approval/permission is required in this study. This study includes no human, animal, or endangered plant samples, and the sample was legally collected in accordance with guidelines provided by the authors’ institution and national or international regulations.
Supplemental Material
Download TIFF Image (70.8 KB)Supplemental Material
Download TIFF Image (251.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/ under the accession no. OR858636. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA1044543, SRR26944584, and SAMN38395455, respectively.
Additional information
Funding
References
- Brummitt RK, Staples GW. 2007. Convolvulaceae. In: Heywood VH, Brummitt RK, Culham A, Seberg O, editors. Flowering plant families of the world. Kew: Royal Botanic Gardens; p. 108–110.
- Brummitt RK. 1963. A taxonomic revision of the genus Calystegia [PhD thesis]. University of Liverpool.
- Chen S, Zhou Y, Chen Y, Gu J. 2018. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 34(17):i884–i890. doi:10.1093/bioinformatics/bty560.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15.
- Eserman LA, Tiley GP, Jarret RL, Leebens-Mack JH, Miller RE. 2014. Phylogenetics and diversification of morning glories (tribe Ipomoeeae, Convolvulaceae) based on whole plastome sequences. Am J Bot. 101(1):92–103. doi:10.3732/ajb.1300207.
- Figueroa-González G, Jacobo-Herrera N, Zentella-Dehesa A, Pereda-Miranda R. 2012. Reversal of multidrug resistance by morning glory resin glycosides in human breast cancer cells. J Nat Prod. 75(1):93–97. doi:10.1021/np200864m.
- Hotta M, Ogata K, Nitta A, Hosikawa K, Yanagi M, Yamazaki K, editors. 1989. Useful plants of the world. Tokyo: Heibonsha Ltd.; p. 196.
- Jin J, Yu W, Yang J, Song Y, dePamphilis C, Yi T, Li D. 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21(1):241. doi:10.1186/s13059-020-02154-5.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780. doi:10.1093/molbev/mst010.
- Lin Y, Li P, Zhang Y, Akhter D, Pan R, Fu Z, Huang M, Li X, Feng Y. 2022. Unprecedented organelle genomic variations in morning glories reveal independent evolutionary scenarios of parasitic plants and the diversification of plant mitochondrial complexes. BMC Biol. 20(1):49. doi:10.1186/s12915-022-01250-1.
- Liu S, Ni Y, Li J, Zhang X, Yang H, Chen H, Liu C. 2023. CPGView: a package for visualizing detailed chloroplast genome structures. Mol Ecol Resour. 23(3):694–704. doi:10.1111/1755-0998.13729.
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274. doi:10.1093/molbev/msu300.
- Ono M, Ichihara Y, Saito N, Yamada M, Yuuki K, Nawata M, Tsutsumi S, Yasuda S, Tsuchihashi R, Okawa M, et al. 2020. Identification and characterization of organic and glycosidic acids in crude resin glycoside fraction from Calystegia hederacea. J Nat Med. 74(1):200–211. doi:10.1007/s11418-019-01366-9.
- Ono M, Saito N, Minamishima H, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Miyashita H, Yoshimitsu H, Nohara T. 2022. Two new glycosidic acids, calyhedic acids E and F, in crude resin glycoside fraction from Calystegia hederacea. Nat Prod Res. 36(1):46–53. doi:10.1080/14786419.2020.1761362.
- Ono M, Takigawa A, Kanemaru Y, Kawakami G, Kabata K, Okawa M, Kinjo J, Yokomizo K, Yoshimitsu H, Nohara T. 2014. Calysolins V–IX, resin glycosides from Calystegia soldanella and their antiviral activity toward herpes. Chem Pharm Bull. 62(1):97–105. doi:10.1248/cpb.c13-00610.
- Ono M, Yuhara N, Shimohara T, Matsubara S, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Zhou JR, Yoshimitsu H, et al. 2021. Calyhedins I–VI: resin glycosides from the rhizomes of Calystegia hederacea. Phytochemistry. 190:112888. doi:10.1016/j.phytochem.2021.112888.
- Pereda-Miranda R, Rosas-Ramírez D, Castañeda-Gómez J. 2010. Resin glycosides from morning glory family. Fortschr Chem Org Naturst. 92:77–153. doi:10.1007/978-3-211-99661-4_2.
- Shi L, Chen H, Jiang M, Wang L, Wu X, Huang L, Liu C. 2019. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Res. 47(W1):W65–W73. doi:10.1093/nar/gkz345.
- Wang K, Li L, Zhao M, Li S, Sun H, Zhang M, Wang Y. 2018. Characterization of the complete chloroplast genome of Jilin ginseng (Panax ginseng C. A. Meyer) using next generation sequencing. Mitochondrial DNA B Resour. 3(2):685–686. doi:10.1080/23802359.2018.1462122.
- Wang Z, Song H, Jiang D. 2021. Complete chloroplast genome sequence of Convolvulus arvensis. Mitochondrial DNA B Resour. 6(7):1814–1815. doi:10.1080/23802359.2021.1915202.
- Wu CS, Chen CI, Chaw SM. 2022. Plastid phylogenomics and plastome evolution in the morning glory family (Convolvulaceae). Front Plant Sci. 13:1061174. doi:10.3389/fpls.2022.1061174.
- Yoshikawa K, Yagi C, Hama H, Tanaka M, Arihara S, Hashimoto T. 2010. Ipomotaosides A–D, resin glycosides from the aerial parts of Ipomoea batatas and their inhibitory activity against COX-1 and COX-2. J Nat Prod. 73(11):1763–1766. doi:10.1021/np100283t.
- Zhang D, Gao F, Jakovlić I, Zou H, Zhang J, Li WX, Wang GT. 2020. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour. 20(1):348–355. doi:10.1111/1755-0998.13096.

