Abstract
The medicinal plant Cyperus brevifolius (Rottb.) Hassk. 1844 has a long history of use in traditional Chinese medicine. In this study, we determined and systematically analyzed the complete chloroplast (cp) genome of C. brevifolius. The genome is 183,717 bp in length with a GC content of 33.24%. It comprises four distinct regions: a large-single copy (LSC) region of 101,190 bp, a small-single copy (SSC) region of 10,366 bp, and two inverted repeat (IR) regions of 36,079 bp each. A total of 137 genes are present in the genome including 89 protein-coding genes, 40 tRNA genes, and eight rRNA genes. Phylogenetic analysis reveals that C. brevifolius belongs to the Cyperus genus. This newly sequenced cp genome provides valuable insights for future genetic and genomic studies on Cyperus.
1. Introduction
Cyperus brevifolius (Rottb.) Hassk. 1844, a widely distributed perennial herb in several provinces of China including Jiangsu, Anhui, Zhejiang, Fujian, Hubei, Sichuan, and Yunnan, exhibits robust vitality and rapid growth. Previous studies have investigated the pharmacological activity of C. brevifolius and demonstrated its hepatoprotective, sedative, digestive, diuretic, sudorific antispasmodic effects as well as its sedative and anticonvulsant properties (Hellión-Ibarrola et al. Citation2016; Chen et al. Citation2020). Additionally, this plant shows potential for biomonitoring and bioremediation (Hao et al. Citation2013; Sarma et al. Citation2017). Despite extensive research on the properties and applications of C. brevifolius in fields such as pharmacology and environmental science researches, its chloroplast (cp) genome remains unexplored. Chloroplasts are vital organelles in plant cells that play a pivotal role in energy conversion and biosynthesis. Their genome encompasses numerous genes associated with photosynthesis and other metabolic processes (Daniell et al. Citation2016). With advancements in sequencing technology from first to third generation, the utilization of cp genomes has become widespread in phylogenetic and species research (Perumal et al. Citation2021). Currently, only 13 cp genomes belonging to Cyperus are available according to the Chloroplast Genome Information Resource (https://ngdc.cncb.ac.cn/cgir/genome?term=Cyperus), which limits molecular identification methods for this genus. Therefore, we aimed to construct a high-quality assembled cp genome of C. brevifolius to facilitate molecular investigations into genetic diversity and phylogenetic relationships.
2. Materials and methods
The young and fresh leaves of C. brevifolius () were collected from Wuhan City, Hubei Province, China (30.48°N, 114.36°E). As C. brevifolius is not categorized as an endangered species, there is no requirement for obtaining permission to collect specimens. The collected specimen has been preserved in the Herbarium of Huanggang Normal University (voucher number 202310HJGC003) and can be accessed through the contact person Xia Fan ([email protected]).
Figure 1. Reference image of the C. brevifolius used in this study. This image was taken by the authors (Xia Fan). The most characteristic feature of the specimen: leaves shorter to slightly longer than culm, mouth obliquely truncate, apex acuminate; spike single, rarely 2 or 3, globose to ovoid-globose, with numerous densely arranged spikelets.

The total genomic DNA was extracted using a modified CTAB method (Allen et al. Citation2006). Subsequently, libraries with an average length of 400 bp were constructed using the Nextera XT DNA Library Preparation Kit (Illumina, San Diego, CA). Library sequencing was performed on the Illumina NovaSeq 6000 platform by Personal Biotechnology (Shanghai, China). The assembly of plastome was conducted using GetOrganelle version 1.7.7 (Jin et al. Citation2020), and the annotation was carried out with GeSeq (Tillich et al. Citation2017). The genome map was generated using CPGview (Liu et al. Citation2023). To determine the phylogenetic position of C. brevifolius, the complete cp genome sequences of 16 species belonged to Cyperus were obtained from NCBI (https://www.ncbi.nlm.nih.gov/), along with Gymnanthemum amygdalinum as an outgroup. Multiple sequence alignment of the entire plastome sequences was performed using MAFFT software (Katoh and Standley Citation2016) under default parameters. A maximum-likelihood (ML) phylogenetic tree based on the Best-fit model of GTR + F + G4 was constructed using IQ-TREE v2.0 (Nguyen et al. Citation2015), and bootstrap replicates were set to 1000. The comparative analysis between C. brevifolius and C. aromaticus, C. michelianus and C. glomeratus was conducted using the mVISTA program (Frazer et al. Citation2004) in a Shuffle-LAGAN mode with default parameters.
3. Results
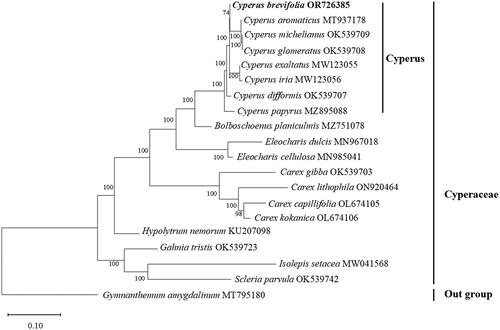
The cp genome size of C. brevifolius (accession number OR726385) was 183,717 bp, with an average depth of ×597.14 (Figure S1). The base composition of this cp genome is as follows: A (33.18%), T (33.58%), C (17.00%), and G (16.24%) resulting in a GC content of 33.24%. The cp genome exhibited a typical quadripartite structure consisting of a large-single copy (LSC) region (101,190 bp), a small-single copy (SSC) region (10,367 bp), and a pair of inverted repeat (IR) regions (36,080 bp) (). In total, there were 137 genes identified in the cp genome of C. brevifolius including 89 protein-coding genes, 40 tRNA genes, and eight rRNA genes. Notably, among these genes were 10 unique cis-splicing genes namely rps16, ycf3, atpF, rpoC1, petB, petD, rpl2, rpl16, ndhA, and ndhB while the gene rps12 was trans-splicing (Figure S2). Furthermore, the phylogenetic relationships between C. brevifolius and other members of Cyperus genus were explored. The results showed that C. brevifolius was belonged to Cyperus genus ().
Figure 2. Genome map of C. brevifolius chloroplast genome was generated using CPGview. The map contains six tracks. From the inner circle, the first track depicts the dispersed repeats connected by red (forward direction) and green (reverse direction) arcs, respectively. The second track shows the long tandem repeats as short blue bars. The third track displays the short tandem repeats or microsatellite sequences as short bars with different colors. The fourth track depicts the sizes of the inverted repeats (IRa and IRb), small single-copy (SSC), and large single-copy (LSC). The fifth track plots the distribution of GC contents along the plastome. The sixth track displays the genes belonging to different functional groups with different colored boxes. The outer and inner genes are transcribed in the clockwise and counterclockwise directions, respectively.
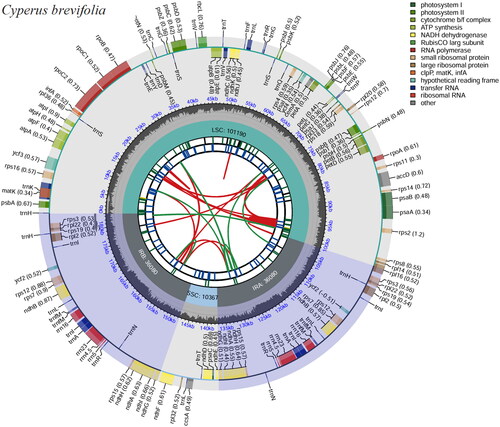
Figure 3. Maximum-likelihood phylogenetic tree of seven related species belonging to Cyperus and the placement of C. brevifolius, 11 species belonging to seven genus of Cyperaceae. Gymnanthemum amygdalinum (Asteraceae) was used as an outgroup. The bootstrap support values are shown on the nodes. The following sequences were used: Cyperus brevifolius OR726385 (this study), Cyperus aromaticus MT937178, Cyperus exaltatus MW123055, Cyperus iria MW123056 (Yang et al. Citation2021), Cyperus difformis OK539707 (Wu et al. Citation2021), Cyperus papyrus MZ895088, Cyperus michelianus OK539709 (Wu et al. Citation2021), Cyperus glomeratus OK539708 (Wu et al. Citation2021), Bolboschoenus planiculmis MZ751078 (Ning et al. Citation2024), Eleocharis dulcis MN967018 (Lee et al. Citation2020), Eleocharis cellulosa MN985041 (Lee et al. Citation2020), Carex gibba OK539703 (Wu et al. Citation2021), Carex lithophila ON920464 (Xu et al. Citation2023), Carex capillifolia OL674105, Carex kokanica OL674106, Hypolytrum nemorum KU207098, Gahnia tristis OK539723 (Wu et al. Citation2021), Isolepis setacea MW041568, Scleria parvula OK539742 (Wu et al. Citation2021), and Gymnanthemum amygdalinum MT795180 (Zhou et al. Citation2021).
In addition, we performed genomic hotspot analysis using mVISTA between C. brevifolius and three species belonged to Cyperus genus (C. aromaticus, C. michelianus, and C. glomeratus). The mVISTA analysis showed that the SSC region was more conserved than the LSC and IR regions (Figure S3). In addition, sequence divergence among all Cyperus was relatively high and mainly concentrated in conserved non-coding sequences and in Untranslated Transcribed Regions (UTR). However, variation among species seemed to be negligible for UTRs located in the IR region. Substitutions were more frequent but indels (trnT-UGU, trnL-UAG) (Figure S3).
4. Discussion and conclusions
Chloroplasts, as the central hub of photosynthesis and energy transduction, play a pivotal role in plant growth and yield (Yu et al. Citation2014). In this study, the complete cp genome of C. brevifolius was sequenced, assembled, and annotated for the first time. The genomic structure of C. brevifolius consists of a pair of IRs, a SSC region, and a LSC region that is similar to the majority of other angiosperms (Liu et al. Citation2022). The phylogenetic analysis revealed that C. brevifolius belongs to Cyperus genus. We analyzed the cp genomes of C. brevifolius and three other Cyperus species, which showed similar genome sizes and CG% content (Table S1). Additionally, among these four species, C. michelianus exhibits the highest total number of genes and CDS genes, while the number of rRNA genes remains consistent across all four species (Table S1).
This study provides valuable molecular insights into elucidating phylogenetic relationships within the Cyperus genus. Furthermore, this genomic resource will facilitate further genetic and genomic investigations, including systematic research advancements, plant exploitation, and resource conservation within the Cyperus genus.
Author contributions
Xia Fan and Yongliang Zheng conceived this project. Kai Yuan analyzed and interpreted the data. Xia Fan drafted and revised the manuscript. All the authors have read and approved the final manuscript.
Ethical approval
C. brevifolius is not classified as endangered or protected, and the collection site does not possess private ownership or protection, thus specific permissions were unnecessary.
Supplemental Material
Download MS Word (80.8 MB)Supplemental Material
Download TIFF Image (44.4 MB)Supplemental Material
Download TIFF Image (69.7 MB)Supplemental Material
Download TIFF Image (9.8 MB)Supplemental Material
Download MS Word (13.3 KB)Supplemental Material
Download MS Word (12.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of the NCBI under the accession number of OR726385 (https://www.ncbi.nlm.nih.gov/nuccore/OR726385.1/). The associated BioProject, Bio-Sample, and SRA numbers are PRJNA1060347, SAMN39225578, and SRR27427975, respectively.
Additional information
Funding
References
- Allen GC, Flores-Vergara MA, Krasynanski S, Kumar S, Thompson WF. 2006. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat Protoc. 1(5):2320–2325. doi:10.1038/nprot.2006.384.
- Chen JF, Wang SM, Nie L. 2020. Effect of Cyperus brevifolia Rottb on epidemic fever, hepatitis and tumor. Asia Pac Tradit Med. 16(6):202–204. doi:10.11954/ytctyy.202006061.
- Daniell H, Lin CS, Yu M, Chang WJ. 2016. Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol. 17(1):134. doi:10.1186/s13059-016-1004-2.
- Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. 2004. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 32(Web Server issue):W273–W279. doi:10.1093/nar/gkh458.
- Hao XQ, Li TX, Zhang XZ, Yu HY, Zhang SJ. 2013. Effect of cadmium on accumulation characteristics of cadmium in the two ecotypes of Kyllinga brevifolia Rottb. J Agro Environ Sci. 32(12):2352–2359.
- Hellión-Ibarrola MC, Montalbetti Y, Heinichen OY, Kennedy ML, Campuzano MA, Alvarenga N, Ibarrola DA. 2016. Antidepressant-like effect of Kyllinga brevifolia rhizomes in male mice and chemical characterization of the components of the active ethyl acetate fraction. J Ethnopharmacol. 194:1005–1011. doi:10.1016/j.jep.2016.10.086.
- Jin JJ, Yu WB, Yang JB, Song Y, dePamphilis CW, Yi TA-O, Li DZ. 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21(1):241. doi:10.1186/s13059-020-02154-5.
- Katoh K, Standley DM. 2016. A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics. 32(13):1933–1942. doi:10.1093/bioinformatics/btw108.
- Lee C, Ruhlman TA, Jansen RK. 2020. Unprecedented intraindividual structural heteroplasmy in Eleocharis (Cyperaceae, Poales) plastomes. Genome Biol Evol. 12(5):641–655. doi:10.1093/gbe/evaa076.
- Liu H, He W, Zhang X, Jiang Z, Li Q, Xia C, Wang H. 2022. Characterization of the complete chloroplast genome of Veronica arvensis and its phylogenomic inference in Plantaginaceae. Mitochondrial DNA B Resour. 7(11):1928–1932. doi:10.1080/23802359.2022.2139162.
- Liu S, Ni Y, Li J, Zhang X, Yang H, Chen H, Liu C. 2023. CPGView: a package for visualizing detailed chloroplast genome structures. Mol Ecol Resour. 23(3):694–704. doi:10.1111/1755-0998.13729.
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ, Tree IQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274. doi:10.1093/molbev/msu300.
- Ning Y, Li Y, Lin HY, Kang EZ, Zhao YX, Dong SB, Li Y, Xia XF, Wang YF, Li CY. 2024. Chromosome-scale genome assembly for clubrush (Bolboschoenus planiculmis) indicates a karyotype with high chromosome number and heterogeneous centromere distribution. Genome Biol Evol. 16(3):evae039. doi:10.1093/gbe/evae039.
- Perumal S, Waminal NE, Lee J, Koo HJ, Choi B, Park JY, Ahn K, Yang TJ. 2021. Nuclear and chloroplast genome diversity revealed by low-coverage whole-genome shotgun sequence in 44 Brassica oleracea breeding lines. Hortic Plant J. 7(6):539–551. doi:10.1016/j.hpj.2021.02.004.
- Sarma H, Islam NF, Prasad MN. 2017. Plant-microbial association in petroleum and gas exploration sites in the state of Assam, north-east India-significance for bioremediation. Environ Sci Pollut Res Int. 24(9):8744–8758. doi:10.1007/s11356-017-8485-8.
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq– versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45(W1):W6–W11. doi:10.1093/nar/gkx391.
- Wu H, Yang JB, Liu JX, Li DZ, Ma PF. 2021. Organelle phylogenomics and extensive conflicting phylogenetic signals in the monocot order Poales. Front Plant Sci. 12:824672. doi:10.3389/fpls.2021.824672.
- Wu RJ, Yu C, Wu Y. 2021. Characterization of the complete plastome of Cyperus rotundus L. Mitochondrial DNA B Resour. 6(1):58–59. doi:10.1080/23802359.2020.1845999.
- Xu S, Teng K, Zhang H, Gao K, Wu J, Duan L, Yue Y, Fan X. 2023. Chloroplast genomes of four Carex species: long repetitive sequences trigger dramatic changes in chloroplast genome structure. Front Plant Sci. 14:1100876. doi:10.3389/fpls.2023.1100876.
- Yang LL, Niu JQ, Tang WW. 2021. The complete chloroplast genome of pioneering plant Cyperus iria L. (Cyperaceae) in ecological restoration. Mitochondrial DNA B Resour. 6(4):1335–1336. doi:10.1080/23802359.2021.1908865.
- Yu Q, Huang C, Yang Z. 2014. Nuclear-encoded factors associated with the chloroplast transcription machinery of higher plants. Front Plant Sci. 5:316. doi:10.3389/fpls.2014.00316.
- Zhou F, Lan K, Li X, Mei Y, Cai S, Wang J. 2021. The complete chloroplast genome sequence of Vernonia amygdalina Delile. Mitochondrial DNA B Resour. 6(3):1134–1135. doi:10.1080/23802359.2021.1902411.
