1. Introduction
Cancer is the main health problem with high morbidity and mortality. The worldwide annual incidence and deaths from cancers are estimated in millions [Citation1,Citation2]. Hence, cancer therapy must be continuously promoted and developed. Cancer study, like in other diseases, highly depends on representative and reliable models. However, the tumor is not uniform, but rather a heterogenic and highly variable and complex than other diseases, rendering its study extremely difficult, expensive [Citation3]. The most common treatment methods for cancers are based on surgery [Citation4], chemotherapy [Citation5], radiotherapy [Citation6], and immunotherapy [Citation7]. The response of cancers to these various treatment strategies differs according to tumor subtype, clinical stage, and associated risk factors and unfortunately fails to limit the progression of cancer in various cases. Even the same tumor of the same organ or tissue differs in response to therapy among patients and the recurrence and metastasis which are associated with high resistance to therapy are main issues [Citation8]. Also, chemotherapy affects the quality of life because of its potential side effects and therefore disfavored by many patients. Therefore, suitable models to expect the treatment response with high precision are of extreme need toward more personalized treatment of patients.
Experimental models are assigned to understand the pathobiology, identify diagnostic and prognostic biomarkers of cancer progression and establish novel potent and effective therapies. To date, the available treatment protocols seem inadequate to prevent the resistance to therapy as well as the recurrence and progression of cancer. Therefore, several experimental models of to study cancer development and progression including rats and mice as well as the in vitro culture models of cell line, 2D cell, induced pluripotent stem cell (iPSCs) lines, spheroids, 3D organoids, organotypic tissue slice cultures, patient-derived tumor xenografts are developed [Citation9–Citation11] and certainly, they confer valuable tools for preclinical pharmacological assessment.
Cancer stem cells (CSCs) are tumor-initiating cells capable of conserving cellular heterogeneity, self-renewal, epithelial to mesenchymal transition, differentiation to form all kind cells in a given tumor [Citation12] and drive the tumor growth, metastasis and most importantly, the resistance to conventional anti-cancer agents [Citation13–Citation22]. Therefore, the total eradication of CSCs is crucial for the successful treatment of cancers. Thus, understanding the mechanism of response and resistance of CSCs to therapy and developing personalized therapy is the cornerstone for treatments of cancer in cancer patients.
Currently, the selection of the correct tool to use in the laboratory to elucidate the mechanism of CSCs’ resistance to therapy can depend on the question on hand, but also on the resources (and knowledge) available. Among them, organoids constitute the more reasonable method in recapitulating the in vivo microenvironment of the tumor, and easier handling as well as high throughput screening than slice cultures or xenografts. During the past decade, researchers have made substantial progress in the field of organoid biology, and organoid systems have already been reviewed in detail elsewhere [Citation21,Citation23,Citation24].
2. Patient-derived organoid analysis of drug resistance in precision medicine
The recent progress in 3D culture engineering has driven to the evolution of novel healthy tissue and cancer models. Upon seeding into a 3D matrix with serum-free media, adult stem cells derived from tissues after digestion can be propagated efficiently into self-organizing organotypic structures, termed organoids. In 2009, Sato et al. firstly established 3D epithelial organoids from a single LGR5+ intestinal stem cell which successfully mimicked the in vivo stem cell niche and grew out as organotypic, highly polarized epithelial structures with proliferative crypt and differentiated villus compartments [Citation25]. This base constructed the starting point for the development of organoid from mouse and human epithelia. Organoids, 3D in vitro cellular structures, are stem cell-derived cultures with the ability to self-organize into ‘mini-copy’ mimicking their original tissue. It can stably recapitulate the in vivo architecture, functions, and genetic and molecular imprint of their parental tissues or organs even after several passaging [Citation26,Citation27]. Additionally, organoids can retain cell-cell and cell-matrix interactions compared with 2D cell culture. It is regarded as a transitional model between cancer cell lines and xenografts [Citation28–Citation30]. This technique holds great promise for use in the medical field for personalized high-throughput drug screening coupled with genomic analysis () not only to predict resistance to anti-cancer drugs but also to identify effective cancer therapy for individual patients [Citation11,Citation31,Citation32]. Several publications on this topic have been carried (), some of which are discussed here.
Figure 1. Preparation patient tumor tissue-derived organoids.
After the tumor specimen is collected, washed in cold phosphate buffer saline, they are minced on ice by a sterilized scissor and collected in a 15 mL tube. Later, they are mechanically and enzymatically digested. Subsequently, the cell suspension passes through a cell strainer to obtain single-cell suspension which is then centrifuged, washed and seeded in Matrigel to form organoids. After several passaging, the organoids can be used for the analysis of drug resistance and sensitivity as well as genomic profiling.
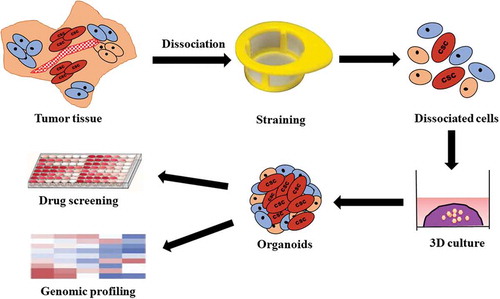
Table 1. Drug screening using organoid technology.
In colorectal cancer (CRC), PDO culture has been used to analyzes the mechanism of drug resistance. Using an air-liquid interface method, Usui et al. [Citation21,Citation33], developed a new CRC organoid culture contained a lot of CSCs and showed resistance to 5-fluorouracil, and irinotecan by activation of hedgehog signaling of CSCs. Moreover, treatment with hedgehog signal inhibitors (AY9944, GANT61) diminished the cell viability of these organoids [Citation21]. In another study, the patient-derived CRC organoids showed high amenability to high-throughput drug screening and allowed personalized therapy design [Citation34]. In this study, they detected resistance to the anti-EGFR inhibitors, cetuximab, and afatinib, in the setting of KRAS mutant organoids, of which, one subset has a BRAF mutation, a well-known mediator of cetuximab resistance [Citation35]. Interestingly, Buzzelli et al. [Citation36], clearly demonstrated that the PDO from liver metastases of CRC retains the chemotherapy resistance characteristics of original tumor isolated from patients with advanced and metastatic CRC and had received three to six cycles of chemotherapy. The organoids showed acquired resistance to oxaliplatin and 5-fluorouracil in a similar manner to the patients.
In bladder cancer, although both 2D and 3D cell culture were used to evaluate the response or resistance to anti-cancer therapy, the 2D culture provided unpredictable, misleading data about in vivo responses probably due to the lack of cell-cell and cell-extracellular matrix signaling that occurs in the 3D culture [Citation37,Citation38]. Human bladder cancer organoids were used efficiently to analyze response and resistance to therapy after they were exposed to various frequently used anti-cancer drugs. Some organoids were relatively resistant to epirubicin and doxorubicin, while others were more sensitive to gemcitabine and vincristine [Citation39]. In dogs, Elbadawy et al. recently demonstrated the difference in response of bladder cancer organoids to commonly used therapy [Citation27]. They showed that treatment with gemcitabine, showed no effect on the cell viability of most of the organoids, except for one. To clarify the difference in gemcitabine sensitivity, the expression levels of genes involved in gemcitabine metabolism (dCK, CDA, RRM1, RRM2, and SLC29A1) were assessed but no correlation was found. Also, individual differences among organoids established from different dogs were observed in response to several patterns of co-treatment with anti-cancer drugs [Citation27].
The PDO of metastatic pancreatic cancer was used also to predict responsiveness or resistance to drugs of primary tumors, as the resistance of the PDO to gemcitabine correlated with the resistance of the original tumors [Citation40].
The PDO of oropharyngeal and esophageal squamous cell carcinoma cells demonstrated resistance to therapy [Citation41]. The formation assay of organoids was directly and significantly correlated with poor response to chemoradiation or presurgical neoadjuvant chemotherapy in patients. They showed that the resistance of organoids to 5-fluorouracil was associated with high CD44 expression and autophagy [Citation41].
The PDO of biliary tract carcinoma was also used to analyze sensitivity and resistance to erlotinib [Citation42]. The erlotinib-highly sensitive organoids are enriched with KLK6 and ANXA10 genes. However, organoids enriched with ALDH3A1 and CPB2 showed resistance to erlotinib.
In liver cancer, the PDO also showed interpatient and intratumor drug response heterogeneity [Citation43]. In this study, 27 liver cancer PDO lines were developed and tested with 129 FDA-approved anti-cancer drugs. The data revealed interpatient and intratumor drug response heterogeneity as cisplatin showed no effect on PDOs, while gemcitabine had a moderate effect. The first-generation proteasome inhibitor, bortezomib, demonstrated high inhibitory effects. After genomic profiling, the organoids showed a mutation in fibroblast growth factor receptor 1 (FGFR1) are more responsive to ponatinib, a multi-kinase inhibitor, while others didn’t show and were resistant [Citation43].
In the PDO of gastric cancer, the patterns of resistance were documented [Citation44]. In this study, PDOs were exposed to 5-FU, irinotecan, oxaliplatin, docetaxel, and epirubicin, and for each drug, various responses were documented. Genomic analysis of the organoids revealed that the irinotecan-resistant organoids carried DD282 mutation, whereas the irinotecan-sensitive ones showed a gene amplification in the ERBB2 gene (HER2/neu) which can be successfully targeted with trastuzumab, an antibody binding to the HER2 receptor.
In summary, the PDO can mimic the typical features of the original tumors. Successful interference with activated pathways in the PDO demonstrates their potential utility in evaluating the response and resistance to therapy for individual patients in precision medicine. On a small scale, comparisons of the patient to organoid therapy responses or resistance have already displayed the high predictive power of PDO. Comprising PDO into clinical trials and testing it along with the patients will ultimately be helpful to predict individual therapy response. In addition, PDO already fills the gap between conventional 2D cell lines-based drug assay and clinical trials.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewers Disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 Mar 1;136(5):E359–86. PubMed PMID: 25220842.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019 Jan;69(1):7–34. PubMed PMID: 30620402; eng. .
- Breitenbach M, Hoffmann J. Editorial: cancer models. Front Oncol. 2018;8:401. PubMed PMID: 30338241; PubMed Central PMCID: PMCPMC6178941. eng. .
- Wyld L, Audisio RA, Poston GJ. The evolution of cancer surgery and future perspectives [Perspective]. Nat Rev Clin Oncol. 2015 Feb;12(2):115–124. PubMed PMID: 25384943. .
- Chabner BA, Roberts TG Jr. Timeline: chemotherapy and the war on cancer. Nat Rev Cancer. 2005 Jan;5(1):65–72. PubMed PMID: 15630416. .
- Thariat J, Hannoun-Levi JM, Sun Myint A, et al. Past, present, and future of radiotherapy for the benefit of patients. Nat Rev Clin Oncol. 2013 Jan;10(1):52–60. PubMed PMID: 23183635.
- Khalil DN, Smith EL, Brentjens RJ, et al. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy [Review Article]. Nat Rev Clin Oncol. 2016 May;13(5):273–290. PubMed PMID: 26977780; PubMed Central PMCID: PMCPMC5551685.
- Nagle PW, Plukker JTM, Muijs CT, et al. Patient-derived tumor organoids for prediction of cancer treatment response. Semin Cancer Biol. 2018 Dec;53:258–264. PubMed PMID: 29966678; eng.
- Bahmad H, Hadadeh O, Chamaa F, et al. Modeling human neurological and neurodegenerative diseases: from induced pluripotent stem cells to neuronal differentiation and its applications in neurotrauma. Front Mol Neurosci. 2017;10:50. . PubMed PMID: 28293168; PubMed Central PMCID: PMCPMC5329035. eng.
- Bahmad HF, Cheaito K, Chalhoub RM, et al. Sphere-formation assay: three-dimensional in vitro culturing of prostate cancer stem/progenitor sphere-forming cells. Front Oncol. 2018;8:347. . PubMed PMID: 30211124; PubMed Central PMCID: PMCPMC6121836. eng.
- Forsythe S, Pu T, Skardal A. Using organoid models to predict chemotherapy efficacy: the future of precision oncology? Expert Rev Precis Med Drug Dev. 2019 Nov Feb;4(6):317–336. .
- Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006 Oct 1;66(19):9339–9344. PubMed PMID: 16990346; eng.
- Bellmunt J. Stem-like signature predicting disease progression in early stage bladder cancer. The role of E2F3 and SOX4. Biomedicines. 2018 Aug 2;6(3):85. PubMed PMID: 30072631; PubMed Central PMCID: PMCPMC6164884. eng. .
- Elbadawy M, Usui T, Yamawaki H, et al. Development of an experimental model for analyzing drug resistance in colorectal cancer. Cancers (Basel). 2018;10(6):6.PubMed PMID: 29843359; PubMed Central PMCID: PMC6025190. eng.
- Usui T, Sakurai M, Nishikawa S, et al. Establishment of a dog primary prostate cancer organoid using the urine cancer stem cells. Cancer Sci. 2017 Dec;108(12):2383–2392. PubMed PMID: 29024204; PubMed Central PMCID: PMCPMC5715251. eng.
- Zhang Y, Wang Z, Yu J, et al. Cancer stem-like cells contribute to cisplatin resistance and progression in bladder cancer. Cancer Lett. 2012 Sep 1;322(1):70–77. PubMed PMID: 22343321; eng.
- Bahmad HF, Mouhieddine TH, Chalhoub RM, et al. The Akt/mTOR pathway in cancer stem/progenitor cells is a potential therapeutic target for glioblastoma and neuroblastoma. Oncotarget. 2018 Sep 11;9(71):33549–33561. PubMed PMID: 30323898; PubMed Central PMCID: PMCPMC6173359. eng.
- Bahmad HF, Chamaa F, Assi S, et al. Cancer stem cells in neuroblastoma: expanding the therapeutic Frontier. Front Mol Neurosci. 2019;12:131. . PubMed PMID: 31191243; PubMed Central PMCID: PMCPMC6546065. eng.
- Tsai YC, Chen WY, Abou-Kheir W, et al. Androgen deprivation therapy-induced epithelial-mesenchymal transition of prostate cancer through downregulating SPDEF and activating CCL2. Biochimica Et Biophysica Acta Mol Basis Dis. 2018 May;1864(5 Pt A):1717–1727. PubMed PMID: 29477409; eng.
- Elbadawy M, Usui T, Yamawaki H, et al. Emerging roles of C-Myc in cancer stem cell-related signaling and resistance to cancer chemotherapy: a potential therapeutic target against colorectal cancer. Int J Mol Sci. 2019 May 11;20(9):2340. PubMed PMID: 31083525; PubMed Central PMCID: PMCPMC6539579. eng.
- Usui T, Sakurai M, Umata K, et al. Hedgehog signals mediate anti-cancer drug resistance in three-dimensional primary colorectal cancer organoid culture. Int J Mol Sci. 2018 Apr 6;19(4):1098. PubMed PMID: 29642386; PubMed Central PMCID: PMCPMC5979580. eng.
- Elbadawy M, Usui T, Yamawaki H, et al. Novel functions of death-associated protein kinases through mitogen-activated protein kinase-related signals. Int J Mol Sci. 2018 Oct 4;19(10):3031. PubMed PMID: 30287790; PubMed Central PMCID: PMCPMC6213522. eng.
- Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science (New York, NY). 2014 Jul 18;345(6194):1247125. PubMed PMID: 25035496; eng. .
- Shamir ER, Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol. 2014 Oct;15(10):647–664. PubMed PMID: 25237826; PubMed Central PMCID: PMCPMC4352326. eng. .
- Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009 May 14;459(7244):262–265. PubMed PMID: 19329995; eng.
- Dutta D, Heo I, Clevers H. disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med. 2017 May;23(5):393–410. PubMed PMID: 28341301. .
- Elbadawy M, Usui T, Mori T, et al. Establishment of a novel experimental model for muscle-invasive bladder cancer using a dog bladder cancer organoid culture. Cancer Sci. 2019 Sep;110(9):2806–2821. PubMed PMID: 31254429; PubMed Central PMCID: PMCPMC6726682. eng.
- Baker LA, Tiriac H, Clevers H, et al. Modeling pancreatic cancer with organoids. Trends Cancer. 2016 Apr;2(4):176–190. PubMed PMID: 27135056; PubMed Central PMCID: PMCPMC4847151. eng.
- Clevers H. Modeling development and disease with organoids. Cell. 2016 Jun 16;165(7):1586–1597. PubMed PMID: 27315476; eng. .
- Jin MZ, Han RR, Qiu GZ, et al. Organoids: an intermediate modeling platform in precision oncology. Cancer Lett. 2018 Feb;1(414):174–180. . PubMed PMID: 29174804; eng.
- Bartfeld S, Clevers H. Stem cell-derived organoids and their application for medical research and patient treatment. J Mol Med (Berl). 2017 Jul;95(7):729–738. PubMed PMID: 28391362; eng. .
- Takahashi T. Organoids for drug discovery and personalized medicine. Annu Rev Pharmacol Toxicol. 2019 Jan 6;59(1):447–462. PubMed PMID: 30113875; eng. .
- Usui T, Sakurai M, Enjoji S, et al. Establishment of a novel model for anticancer drug resistance in three-dimensional primary culture of tumor microenvironment. Stem Cells Int. 2016;2016:7053872. . PubMed PMID: 28119740; PubMed Central PMCID: PMCPMC5227181.
- van de Wetering M, Francies HE, Francis JM, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015 May 7;161(4):933–945. PubMed PMID: 25957691; PubMed Central PMCID: PMCPMC6428276.
- Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008 Dec 10;26(35):5705–5712. PubMed PMID: 19001320; eng.
- Buzzelli JN, Ouaret D, Brown G, et al. Colorectal cancer liver metastases organoids retain characteristics of original tumor and acquire chemotherapy resistance. Stem Cell Res. 2018 Mar;27:109–120. PubMed PMID: 29414601; PubMed Central PMCID: PMCPMC5842239. eng.
- Bodgi L, Bahmad HF, Araji T, et al. Assessing radiosensitivity of bladder cancer in vitro: A 2D vs. 3D approach. Front Oncol. 2019;9:153. . PubMed PMID: 30941305; PubMed Central PMCID: PMCPMC6433750. eng.
- Breslin S, O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today. 2013 Mar 01;18(5):240–249.
- Mullenders J, de Jongh E, Brousali A, et al. Mouse and human urothelial cancer organoids: A tool for bladder cancer research. Proc Natl Acad Sci U S A. 2019 Mar 5;116(10):4567–4574. PubMed PMID: 30787188; PubMed Central PMCID: PMCPMC6410883.
- Choi SI, Jeon AR, Kim MK, et al. Development of patient-derived preclinical platform for metastatic pancreatic cancer: PDOX and a subsequent organoid model system using percutaneous biopsy samples [original research]. Front Oncol. 2019 Sept 13;9(875):875. PubMed PMID: 31572675; PubMed Central PMCID: PMCPMC6753223. English.
- Kijima T, Nakagawa H, Shimonosono M, et al. Three-dimensional organoids reveal therapy resistance of esophageal and oropharyngeal squamous cell carcinoma cells. Cell Mol Gastroenterol Hepatol. 2019;7(1):73–91. . PubMed PMID: 30510992; PubMed Central PMCID: PMCPMC6260338. eng.
- Saito Y, Muramatsu T, Kanai Y, et al. Establishment of patient-derived organoids and drug screening for biliary tract carcinoma. Cell Rep. 2019 Apr 23;27(4):1265–1276 e4. PubMed PMID: 31018139; eng.
- Li L, Knutsdottir H, Hui K, et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI Insight. 2019 Jan 24;4(2). PubMed PMID: 30674722; PubMed Central PMCID: PMCPMC6413833. DOI:10.1172/jci.insight.121490.
- Seidlitz T, Merker SR, Rothe A, et al. Human gastric cancer modelling using organoids. Gut. 2019 Feb;68(2):207–217. PubMed PMID: 29703791; PubMed Central PMCID: PMCPMC6352409.
- Schutte M, Risch T, Abdavi-Azar N, et al. Molecular dissection of colorectal cancer in pre-clinical models identifies biomarkers predicting sensitivity to EGFR inhibitors. Nat Commun. 2017 Feb 10;8(1):14262. PubMed PMID: 28186126; PubMed Central PMCID: PMCPMC5309787. eng.
- Tashiro T, Okuyama H, Endo H, et al. In vivo and ex vivo cetuximab sensitivity assay using three-dimensional primary culture system to stratify KRAS mutant colorectal cancer. PloS One. 2017;12(3):e0174151. . PubMed PMID: 28301591; PubMed Central PMCID: PMCPMC5354432. eng.
- Kondo J, Ekawa T, Endo H, et al. High-throughput screening in colorectal cancer tissue-originated spheroids. Cancer Sci. 2019 Jan;110(1):345–355. PubMed PMID: 30343529; PubMed Central PMCID: PMCPMC6317944. eng.
- Lee SH, Hu W, Matulay JT, et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. 2018 Apr 5;173(2):515–528 e17. PubMed PMID: 29625057; PubMed Central PMCID: PMCPMC5890941. eng.
- Yan HHN, Siu HC, Law S, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018 Dec 6;23(6):882–897 e11. PubMed PMID: 30344100; eng.
- Pauli C, Hopkins BD, Prandi D, et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 2017 May;7(5):462–477. PubMed PMID: 28331002; PubMed Central PMCID: PMCPMC5413423. eng.
