1. Introduction
Acute myelogenous leukemia (AML) is a malignant disease of the blood and bone marrow, characterized by proliferation, lack of apoptosis, and block in differentiation of leukemic blasts [Citation1]. These immature cells compromise normal bone marrow function, resulting in severe bone marrow failure, with anemia, thrombocytopenia, and granulocytopenia [Citation1]. The disease has a highly malignant and aggressive course, and without treatment the patients will usually die within weeks to months. Except for promyelocytic AML, the only possibility for a longtime cure is intensive chemotherapy, and in high-risk patients combined with allogenic hematopoietic stem cell transplantation (allo-HSCT) [Citation1,Citation2]. Allo-HSCT is the most potent antileukemic treatment, by utilization of the immune mediated graft versus leukemia (GVL) effect derived from immune competent donor cells. However, allo-HSCT is associated with a high degree of morbidity and mortality, especially due to severe graft versus host disease (GVHD). Furthermore, the occurrence of relapse of the disease even after transplantation is still prevalent, especially for patients present with genetic markers associated with inferior prognosis at the time of diagnosis [Citation2,Citation3]. In the following, we will discuss the current and future aspects of allo-HSCT in the setting of AML, untangling the possibilities to optimize the treatment approaches based on precision medicine.
1.1. Allo-HSCT in the setting of AML therapy-state of the art
The standard therapies for younger and fit AML patients have been induction therapy with an anthracycline and cytarabine, the 3 + 7 regimen [Citation2,Citation4]. This regimen leads to morphological complete remission (CR) in approximately 60%-80% of younger adults and in 40%-60% of older adults (>60 years) [Citation2]; however, the majority of patients will relapse without additional therapy. Recent decades of research have brought into light the fact that AML is a very heterogeneous disease, and from a treatment point of view, this is particularly well reflected in terms of differences in cytogenetic and molecular genetic changes [Citation1]. Post remission strategies comprise intensive chemotherapy and high-dose therapy followed by autologous- (auto-) or allo-HSCT. The choices of strategies relay on prognostic scoring systems based on cytogenetics, molecular genetics and treatment response criteria, although they so far do not involve specific targeted therapy for subgroups of patients [Citation2,Citation4]. Generally, this means post-remission therapy by allo-HSCT is recommended in fit patients when the risk of recurrence is in excess of 35–40%, and thus exceeds the treatment-related mortality (TRM) rate associated with allo-HSCT [Citation1,Citation2].
2. Improving treatment approaches for individual patients
Although recent years have seen the rise of new pharmacological treatment options for patients with AML [Citation4], this has mainly provided alternatives to achieve remission, and the hope for curation of the majority of patients still relies on the possibility to undergo allo-HSCT. However, given the high TRM rates, the potential benefits must be carefully weighed up against potential harm for the individual patient. A precision medicine approach regarding allo-HSCT is currently important and will likely be even more so in the future. This is reflected through assessments made in all parts of the time course related to allo-HSCT (). We will further discuss current and future challenges related to the various parts, based on a precision medicine approach.
Figure 1. The specific phases in the allo-HSCT course, and how different approaches can be used to intervene in different phases. Abbreviations: MRD, measurable residual disease
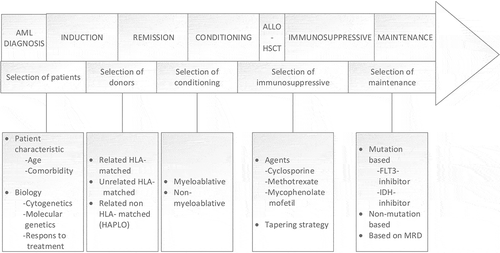
2.1. Selection of patients
The first step in allo-HSCT procedure is the selection of patients considered candidates for a transplant. This is based on both patient characteristics, mainly patients age and comorbidities, and disease biology. The former is based on general consideration by the treatment physicians, although it can be further standardized by use of objective scoring system like the hematopoietic cell transplantation-comorbidity index (HCT-CI) [Citation5]. The last is currently based on cytogenetic and molecular genetic features, and the European Leukemia Net (ELN) 2017 recommendations are currently the most used prognostic scoring system [Citation2]. This divides patients into three major prognostic categories: favorable, intermediate and adverse, where the two last groups in general should be offered allo-HSCT in first CR, if a suitable donor is identified [Citation2]. Only six molecular mutations are currently incorporated in the classification system; namely NPM1, CEBPA, FLT3, RUNX1, ASXL1 and TP53 [Citation2]. However, on average an AML genome harbors 13 molecular mutations [Citation6], and further studies are needed to clarify the prognostic impact of different mutations and combination of different mutations. Furthermore, several mutations are currently being druggable by novel therapeutic agents, although whether new agents offer subgroups of patients a better chance of cure without allo-HSCT, must be investigated in further and larger studies.
2.2. Selection of donor
Selection of donors is based on compatibility in the human leukocytes antigen (HLA) system, and traditionally a better match, i.e. 10/10 or 12/12 HLA match has usually been preferred, as this has been associated with a decreased risk of GVHD. Usually, the donor search starts with identifying HLA match among siblings, and if this opportunity is ruled out, the search for a HLA matched unrelated donor (MUD) should be initiated. However, it has been known for decades that increasing GVHD also come along with an increased GVL effect, i.e. patients with established GVHD have a decreased risk of developing relapsed disease [Citation7].
The concept of HLA mismatches in allo-HSCT has been the subject of intense debate, however accumulating evidence suggests limited alloreactivity is sufficient for GVL, while counter wise high alloreactivity can lead to clinically uncontrollable GVHD. Hence the term permissive mismatches, for eliciting limited alloreactivity, and hence shifting balance in favor of GVL compared to GVHD, has been introduced. This is most relevant in MUD transplant, there HLA-DP, permissive HLA-DP mismatches which mediate limited T-cell alloreactivity and thereby shift the balance from GVHD toward GVL. However, if selected special high-risk AML patients, i.e. patients with relapse or refractory disease or patients with complex karyotype or TP53 mutations, should be offered an allo-HSCT based on permissive mismatches remains open, and should be explored in further clinical trials.
In recent years, the concept of haploidentical transplantation for patients without HLA-matched donors has been introduced [Citation8]. Interestingly, haploidentical transplant seems to have comparable clinical outcomes compared to both matched and unmatched allo-HSCT [Citation9]. The incidence of GVHD seems not to be significantly increased with post-transplant cyclophosphamide GVHD prophylaxis, and there are some indications that cytotoxic T-lymphocytes derived from haplo-HSCT have higher cytotoxicity [Citation10], with the potential to reduce the risk of relapsed disease [Citation11].
2.3. Selection of conditioning regimen
For simplification, the preparative regimen could be of two categories, myeloablative conditioning (MAC) and reduced intensive conditioning (RIC) [Citation3]. The first one is associated with a significant increase in relapse free survival [Citation3], although comes with an increased risk of toxicity and TRM [Citation3]. RIC is hence often offered older and more comorbid patients. However, probably should a more personalized medicine approach also be taken into account when choosing the conditioning regimen. For the AML cohort, the main challenge will be selecting younger, fitter patients who benefit from RIC without experience an excessive relapse risk, and on the counter side selecting patients with such a higher degree of relapse risk that they should be offered a MAC regimen [Citation3]. This is clearly demonstrated for patients with a special inferior poor prognosis, for instance patients harboring a TP53 mutation have a very dismal prognosis, and a MAC regimen will probably offer these patients a better chance of survival [Citation12]. Finally, the role of minimal/measurable residual disease (MRD) seems to play an increasingly important role, as evidence supports that MAC instead of RIC reduces risk of relapse and increases survival for patients who are molecular MRD positive before transplantation [Citation13].
3. Selection of immunosuppressive strategies
Immunsuppressiv treatment is regarded mandatory after allo-HSCT, to reduce the risk of engraftment failure and GVHD. Cyclosporine, methotrexate and mycophenolate mofetil are the most common used agents [Citation14]. However, immunosuppressive treatment also reduce the GVL effect, and hence potential increase the risk of relapsed disease [Citation7]. Therefore, in the future, the immunosuppressive strategies post-transplant should probably in higher degree be relied on a personalized medicine approach, where the patients with high risk of relapse, i.e. adverse prognosis, should be offered less immunosuppressive prophylaxis. This is somehow already taken into account, by recommendation of more rapidly tapering of immunosuppressive approaches if the risk of relapse is considered high [Citation7]. Finally, strategies reducing the risk of GVHD, without reducing the GVL effect are strongly desired. A recent publication suggest antithymocyte globulin (ATG) may possess such properties, as it could reduce the risk of GVHD without compromising with increased risk for relapse [Citation15].
3.1. Selection of maintenance therapy and post transplantation monitoring
The use of maintenance treatment after allo-HSCT has been of debate for some years. Different pharmacological strategies, such as hypomethylating agents, histone deacetylase and hedgehog inhibitors and finally FLT3 inhibitors have all been investigated (). The benefits were generally rather disappointing for most entities and general recommendation is warranted regarding this subject. However, recent years’ research has brought renewed interest to this field, and studies have demonstrated encouraging results both regarding hypomethylating agents, histone deacetylases and hedgehog inhibitors and, probably so far most successfully, regarding FLT3 inhibitors (). Maintenance therapy with sorafenib, a multitargeted and FLT3-kinase inhibitor, reduced the risk of relapse and death after allo-HSCT for the subgroup of patients with FLT3-ITD–positive AML [Citation16]. Further and larger randomized trials should try to answer the questions related to which treatment approach should be offered different subgroups. Of special interest are patient with favorable genetic risk profile, i.e. NPM1 mutations or t(8,21)/RUNX1-RUNX1T1 AML, where molecular MRD monitoring during the course of consolidation therapy can predict risk of relapse disease, and hence these patients can befit from early allo-HSCT strategy [Citation17–19]. Furthermore, the potential role of MRD [Citation20–22], in selecting patients for utterly interventions should be further explored. From the perspective of precision medicine, MRD monitoring will probably become even more important in the future, and to an even greater extent be based on individual genetic patient profiles, with increasing possibility of early and targeted initiation of treatment approaches [Citation4,Citation19]. This also include pharmacological targeting of other potential mutations, i.e. IDH-inhibitors for cases with IDH1 or IDH2 mutations found in approximately 10–15% of AML patients [Citation4]. However, at the present, uncertainties related to; appropriate timing, material and method approaches, and treatment implications, are significant in such respects [Citation19].
Table 1. Pharmacological interventions after allo-HSCT for AML
4. Conclusion and future perspective
Precision medicine has already entered the treatment approach of AML and allo-HSCT, although we have probably so far only seen the beginning of a new era, in which the importance of these principles will only become more important. Given the heterogeneity of AML, where identifying subgroups of patients based on genetic mutations, and individual treatment approaches are highly warranted. Although, at the same time, this is challenging, regarding the difficulties to conduct clinical trials, given that all the aspects of the course in allo-HSCT can be manipulated. This should be taken into account for the design of future clinical trials. Currently we are at the beginning of an era where a more precision medical approach is emerging in the setting of allo-HSCT and AML, and we will hopefully substantially improve treatment outcomes for this highly deadly disease.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018 Aug 18;392(10147):593–606.
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017 Jan 26;129(4):424–447.
- Scott BL, Pasquini MC, Logan BR, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017 Apr 10;35(11):1154–1161.
- Reikvam H, Hemsing AL, Smith C. Therapy for acute myelogenous leukemia revisited: moving away from a one-size-fits-all approach. Expert Rev Anticancer Ther. 2020 Oct;25:1–4.
- Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005 Oct 15;106(8):2912–2919.
- Ley TJ, Miller C, Ding L, et al. Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013 May 30;368(22):2059–2074.
- Weiden PL, Flournoy N, Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979 May 10;300(19):1068–1073.
- Ma L, Han X, Jiang S, et al. Haploidentical stem cell transplantation vs matched unrelated donor transplantation in adults with hematologic malignancies: a systematic review and meta-analysis. Hematology. 2020 Dec;25(1):356–365.
- Wang Y, Liu QF, Xu LP, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015 Jun 18;125(25):3956–3962.
- Guo H, Chang Y-J, Hong Y, et al. Dynamic immune profiling identifies the stronger graft-versus-leukemia (GVL) effects with haploidentical allografts compared to HLA-matched stem cell transplantation. Cell Mol Immunol. 2021. DOI: 10.1038/s41423-020-00597-1. Epub ahead of print.
- Chang YJ, Wang Y, Liu YR, et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J Hematol Oncol. 2017 Jul 4;10(1):134.
- Ciurea SO, Chilkulwar A, Saliba RM, et al. Prognostic factors influencing survival after allogeneic transplantation for AML/MDS patients with TP53 mutations. Blood. 2018 Jun 28;131(26):2989–2992.
- Hourigan CS, Dillon LW, Gui G, et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J Clin Oncol. 2020 Apr 20;38(12):1273–1283.
- Penack O, Marchetti M, Ruutu T, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020 Feb;7(2):e157–e167.
- Chang YJ, Wu DP, Lai YR, et al. Antithymocyte globulin for matched sibling donor transplantation in patients with hematologic malignancies: a multicenter, open-label, randomized controlled study. J Clin Oncol. 2020 Oct 10;38(29):3367–3376.
- Burchert A, Bug G, Fritz LV, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol. 2020 Sep 10;38(26):2993–3002.
- Zhu HH, Zhang XH, Qin YZ, et al. MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: results from the AML05 multicenter trial. Blood. 2013 May 16;121(20):4056–4062.
- Balsat M, Renneville A, Thomas X, et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: a study by the acute leukemia French association group. J Clin Oncol. 2017 Jan 10;35(2):185–193.
- Jentzsch M, Schwind S, Bach E, et al. Clinical challenges and consequences of measurable residual disease in non-APL acute myeloid leukemia. Cancers . Basel. Vol. 11. Oct 23; 2019. p. 11.
- Short NJ, Ravandi F. How close are we to incorporating measurable residual disease into clinical practice for acute myeloid leukemia? Haematologica. 2019 Aug;104(8):1532–1541.
- Thol F, Gabdoulline R, Liebich A, et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood. 2018 Oct 18;132(16):1703–1713.
- Heuser M, Gabdoulline R, Löffeld P, et al. Individual outcome prediction for myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia from MDS after allogeneic hematopoietic cell transplantation. Ann Hematol. 2017 Aug 1;96(8):1361–1372.
- Oran B, De Lima M, Garcia-Manero G, et al. Maintenance with 5-azacytidine for acute myeloid leukemia and myelodysplastic syndrome patients. Blood. 2018;132(Supplement 1):971.
- De Lima M, Oran B, Champlin RE, et al. CC-486 maintenance after stem cell transplantation in patients with acute myeloid leukemia or myelodysplastic syndromes. Biol Blood Marrow Transplant. 2018 Oct;24(10):2017–2024.
- Pusic I, Choi J, Fiala MA, et al. Maintenance therapy with decitabine after allogeneic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2015 Oct;21(10):1761–1769.
- Bug G, Burchert A, Wagner EM, et al. Phase I/II study of the deacetylase inhibitor panobinostat after allogeneic stem cell transplantation in patients with high-risk MDS or AML (PANOBEST trial). Leukemia. 2017 Nov;31(11):2523–2525.
- Kent A, Vasu S, Schatz D, et al. Glasdegib as maintenance therapy for patients with AML and MDS patients at high risk for postallogeneic stem cell transplant relapse. Blood Adv. 2020 Jul 14;4(13):3102–3108.
- Schlenk RF, Weber D, Fiedler W, et al. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood. 2019 Feb 21;133(8):840–851.
- Sandmaier BM, Khaled S, Oran B, et al. Results of a phase 1 study of quizartinib as maintenance therapy in subjects with acute myeloid leukemia in remission following allogeneic hematopoietic stem cell transplant. Am J Hematol. 2018 Feb;93(2):222–231.
- Levis MJ, Hamadani M, Logan B, et al. A phase 3, trial of gilteritinib, as maintenance therapy after allogeneic hematopoietic stem cell transplantation in patients with FLT3-ITD+ AML. J Clin Oncol. 2018;36(15suppl):Abstract7075.
