1. Introduction
The immune system has a fundamental role in controlling the development and progression of tumors, including those originating in the bladder, kidney and prostate. However, malignant cells and cells of the tumor microenvironment can induce immune escape mechanisms, including activation of immune checkpoints such as programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) [Citation1,Citation2]. Based on such information, new immune therapies, including inhibition of PD-1 and PD-L1, have been developed recently. Such therapies are at present recommended as standard treatment in patients with metastatic bladder and kidney cancer. In addition to its therapeutic target, PD-L1 appears to be a prognostic parameter. However, data accumulated so far on its predictive role are still controversial.
Little is known about the signaling pathways that induce PD-L1 expression in cancer cells and its functional role in malignant cells [Citation1,Citation2]. Distinct mechanisms can regulate PD-L1 expression in tumors [Citation2]. ‘The understanding of the complex biological function of PD-L1 will improve therapeutic options in urological malignancies’ [Citation1].
Immune checkpoint inhibitors (ICIs) targeting the interaction of PD-1 and CTLA-4 with their specific ligands have been successful in a wide range of solid tumors, dramatically changing the therapeutic landscape of several genitourinary malignancies. However, the identification of specific molecular and histological biomarkers, which could guide treatment selection and could be predictive of response to ICIs, represents one of the hottest areas of research in this setting. Currently, high expression of PD-L1 by immunohistochemistry has been associated with response to PD-1/PD-L1 antibodies and better clinical outcomes in a number of cancer types [Citation3]. However, the role of this test is highly debated due to several reasons, including the lack of specific guidelines regarding the evaluation of PD-L1, remarkable differences in scoring systems in registration studies and the components of PD-L1 assessment – such as tumor cells or immune cells.
2. Distinct mechanisms regulating PD-L1 expression
copy number alterations/amplifications/translocation
transcription factors
oncogenic pathway activation
cytotoxic agents/chemotherapeutics
miRNAs and lncRNAs
posttranslational modifications
3. Immunotherapy and bladder cancer
The US Food and Drug Administration (FDA) has recently approved five ICIs for the treatment of locally advanced or metastatic urothelial carcinoma (mUC) of the bladder and the upper urinary tract: the PD-L1 inhibitors atezolizumab, avelumab and durvalumab and the PD-1 inhibitors pembrolizumab and nivolumab. However, since approximately one-third of patients respond to ICIs and an even smaller proportion of subjects achieve durable responses, the identification of predictive biomarkers of response is a crucial need in this setting [Citation4]. In fact, the mechanisms underlying variation in tumor response to ICIs in mUC patients are far from being fully elucidated. Thus, the identification of predictive biomarkers which could help clinicians in treatment selection and in distinguishing responders to non-responders is an urgent need in this setting. Moreover, atezolizumab and pembrolizumab received approval for first-line treatment of patients that are ineligible for cisplatin whose tumors are positive for PD-L1 expression assessed by immunohistochemistry [Citation5]. Several emerging issues on antibodies, assays, platforms and scoring algorithms have raised concerns about the comparability and interchangeability between these tests [Citation6]. Hence, the two trials that led to the approval of atezolizumab (IMvigor210) and pembrolizumab (KEYNOTE-052) used different cutoffs and methods; as regards the former, the study used the VENTANA SP142 immunohistochemistry assay to evaluate PD-L1 expression on tumor infiltrating immune cells (IC), with scoring criteria reporting IC0, IC1, or IC2/3 according to PD-L1 expression <1%, ≥1% and <5%, and ≥5% of ICs, respectively. Conversely, the KEYNOTE-052 used the 22C3 pharmDx assay to investigate PD-L1 expression, with positive tumors presenting PD-L1 combined positive score (CPS) ≥10 [Citation5]. The CPS is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100 (). Although the efficacy of immunotherapy has been demonstrated, the fraction of MIBC patients who are sensitive to immunotherapy is limited. In order to select candidates for immunotherapy strategies, some biomarkers other than PD-L1 expression are being investigated such as tumor mutational burden (TMB), tumor-infiltrating lymphocytes (TILs), and molecular subtypes. Molecular subtypes may provide independent and complementary information for predicting immunotherapy response. Basal subtype tumors appear to be more sensitive to immunotherapy compared to luminal subtype [Citation7]. ‘Hot’ tumors defined as tumors with high TMB, and CD8 + T cells infiltration are reported to have the highest response with immunotherapy [Citation8].
Figure 1. Urothelial carcinoma showing strong and diffuse expression for PD-L1 on tumor cells assessed by immunohistochemistry with the antibody 22C3 Dako (20x)
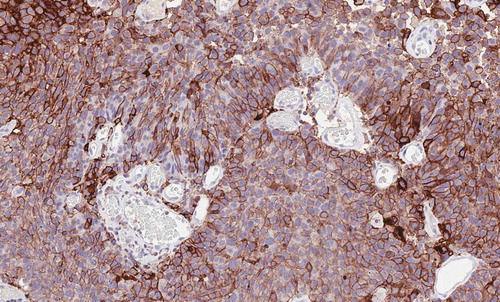
Concerning immune checkpoint inhibitors in the adjuvant or neoadjuvant setting, or in combination with chemotherapy, is still a matter of debate but some encouraging results support their future use in clinical practice. Despite extensive genomic research and multiple combinations of biomarkers, a clinically applicable test to determine the optimal treatment for patients has not been elucidated so far.
4. Immunotherapy and prostate cancer (PCa)
Despite PCa has been traditionally considered an immunologically ‘cold’ malignancy, with not encouraging preliminary findings in early trials, the interactions between immune system and PCa have been extensively studied and are well-known, as witnessed by sipuleucel-T, an autologous vaccine representing the only FDA-approved immunotherapy for PCa. However, trials investigating anti-PD-L1 agents such as atezolizumab, avelumab and durvalumab, alone or in combination with other immunotherapies, chemotherapy or loco-regional treatments are currently ongoing, and preliminary results have been recently published [Citation9,Citation10]. Interestingly, while several studies reported disappointing results, few studies, such as Keynote 199, showed that ‘while responses to pembrolizumab in metastatic castration-resistant PCa may be few, responses can be durable’ regardless of PD-L1 status [Citation11]. Nonetheless, the exploration of potential biomarkers able to identify responders is warranted, despite initial results have indicated that PD-L1 status alone could not be a proper predictive biomarker in this setting. PCa has been shown to have infiltrating lymphocytes mainly represented by T-regulatory (Treg) and T helper 17 (Th17) phenotypes, which suppress autoreactive T cells and antitumor immune responses [Citation12]. Androgen deprivation therapy (ADT) has been reported to temporarily mitigate T cell tolerance and induce T cell activation and migration. Immune-based combinations with cytotoxic chemotherapy or ADT may have the potential to increase response rates in subgroups of non-metastatic castration-resistant PCa patients by inducing expression of PD-1 and PD-L1. Numerous trials are investigating different combinations and results are expected soon to better define if there is a role – and what kind of role – for immunotherapy in this tumor.
5. Immunotherapy and renal cell carcinoma
Similarly, to mUC, the predictive value of PD-L1 expression for response to immunotherapy remains controversial in renal cell carcinoma (RCC) with several issues about PD-L1 and deserve discussion [Citation13,Citation14]. Among these, PD-L1 expression has been reported to be heterogeneous not only between primary tumor and secondary sites, but also within the same primary lesion. In addition, as in the case of mUC patients, the ideal cutoff to define PD-L1 negativity or positivity is matter of debate, with several treatments – including radiotherapy (RT) and tyrosine kinase inhibitors (TKIs) – which have been reported to modify this value. Moreover, methodological and technical issues persist, with several antibodies, platforms, and scoring systems used and no standardization of these methods [Citation14]. Probably, rather than a single biomarker, a crucial point may be to assess different molecular and clinical predictors, which are currently under investigation. However, no association with tumor mutation burden, gene expression associated with immune response, frameshift/small insertion, deletions and clinical utility was found in recently published phase III clinical trials. Combinations of different biomarkers and gene signatures associated with angiogenesis, immune response and myeloid inflammatory infiltrates are promising biological features and need to be validated in the context of prospective clinical trials. Subgroup analysis of large multicenter clinical trials suggest that ICI may have significant clinical activity in sarcomatoid and rhabdoid RCC. Tumors with sarcomatoid/rhabdoid histological features had longer OS and PFS and a higher ORR/CR rate when treated with ICIs compared to VEGF and mTOR targeted therapies suggesting distinctive molecular properties underlying these subtypes. Recently, Bakouny et al. reported that these tumors exhibit an immune-inflamed phenotype characterized by immune activation, increased cytotoxic immune infiltration, upregulation of antigen presentation machinery genes, and PD-L1 expression [Citation15].
Multiple trials are now investigating the role of ICIs in non-clear cell RCC (ncc-RCC). Overall, advanced nccRCC patients treated with first-line ICI experienced improved OS compared to VEGF and mTOR therapy treated patients [Citation16].
6. Immunotherapy and testicular and penile tumors
Testes are considered immunologically privileged sites due to their peculiar immunological environment that protects germ cells from autoimmune damage and to a lack of response of the testicular immune system to antigens. This immune privilege is thought to be driven by a constitutive expression of PD-L1 in normal testis [Citation17]. However, subsequent publications reported contrasting results. Fankhauser et al. reported high levels of PD-L1 expression in 73% seminomas and 64% non-seminomatous tumors evaluated with no expression detected in any of the normal tissue specimens or precursor germ cell neoplasia in situ (GCNIS) lesions [Citation18]. Moreover, Cierna et al. showed that high PD-L1 expression in primary tumor tissue correlated with poor-risk clinical characteristics while patients with low PD-L1 expression showed a better progression-free survival and overall survival, thus proving the poor prognostic value of PD-L1 expression in testicular tumors [Citation19]. So far, data on the efficacy of ICIs are reported in three phase 2 clinical trials investigating pembrolizumab, avelumab and durvalumab, alone or in combination with tremelimumab (anti-CTLA–4 inhibitor). Overall, the results are discouraging since most patients experienced disease progression [Citation20–22].
In penile cancer, PD-L1 expression has been reported in 62.2% of primary tumors with a strong positive correlation with metastatic samples. This provided rational support for application of immunotherapy in penile squamous cell carcinoma [Citation23]. Results are awaited from the clinical trials investigating pembrolizumab (NCT02837042) and ipilimumab plus nivolumab for advanced rare tumors including penile cancer (NCT03333616, NCT02834013).
7. Combination therapies
A new frontier of great interest in genitourinary malignancies is based on the combination of ICIs with other investigational or established anticancer strategies, an approach which has provided remarkable results especially in metastatic RCC (mRCC) [Citation24]. Combination strategies have been developed to bypass de novo or adaptive immune resistance mechanisms that can come up with monotherapies, aiming at a synergistic antitumor effect. These combination therapies are based on strong preclinical rationales. In fact, as regards the combination of immunotherapy with anti-VEGF agents, hypoxia supports malignant cell escape from mechanisms of immune surveillance, and thus impairs the physiological action of immune effector cells. Additionally, recent reports have shown an association between the activation of hypoxia-inducible factor 1 alpha and the upregulation of PD-L1 expression. Since antiangiogenic agents enhance hypoxia and hypoxia areas are associated with PD-L1, anti-VEGF drugs improve T-cell activity. The interaction of angiogenesis and immunosuppression seems to facilitate tumor development and progression [Citation25].
In addition, some combinations include either different immunotherapies targeting distinct immune pathways such as agents aiming to reverse T cell dysfunction; agents targeting other immune inhibitory pathways, such as inhibitors of indoleamine dioxygenase, regulatory T cells, and myeloid-derived suppressor cells [Citation26]; type I interferon, toll-like receptor agonists, vaccines, and oncolytic viruses enhancing antigen presentation – or immunotherapies with other therapeutic approaches, such as targeted agents, radiotherapy and chemotherapies.
Overall, immune-based combinations including the dual checkpoint blockade or based on the combination of an ICI with targeted agents have revolutionized the therapeutic landscape of treatment-naïve RCC [Citation27]. In fact, international guidelines support the use of immune-based combinations in front-line setting, based on the recent results of several phase III trials – including the CheckMate 214, the Keynote-426, and the recently presented CheckMate 9–ER [Citation28–31].
In particular, as regards the former, this study comparing the combination of nivolumab plus ipilimumab versus sunitinib as first-line treatment for mRCC patients has provided unprecedented results, with a complete response rate of 10% – which has been also confirmed by extended follow-up reports *** [Citation32]***.
In 2019, FDA approved pembrolizumab plus axitinib and avelumab plus axitinib for metastatic and advanced RCC, thus giving support to the potential for enhanced activity when combining ICIs with multi-targeted TKIs (i.e. an axitinib drug) [Citation33].
In the therapeutic scenario of mRCC, RT is gaining attention since novel administration techniques are indicating that this tumor is not radioresistant [Citation24,Citation25]. The systemic immune effects of RT can be enhanced by the combination with ICIs that increase the immune response. Some limitations that need to be better defined are the best timing of these two treatments administration and the optimal dose and fractionation scheme of RT. Several ongoing clinical trials will assess if combination strategies can overcome radio-resistance and immunosuppression that can impair the response to immunotherapy.
Clinical studies are currently investigating ICIs together with multi-targeted TKIs in metastatic/advanced urothelial cancer. Preliminary data show results that appear to be promising. In PCa, the combinations of ICIs with tumor-targeted vaccine or cytokines are also considered an interesting field of clinical investigation.
8. Conclusions
The concept that the immune response has a key role in the proliferation of tumor cells represents an important resource in the therapy of cancer. Promising approaches have been adopted to overcome the capacity of cancer cells to inhibit the immune surveillance. This would otherwise defend the host from development and/or tumor progression of cancers.
Immune checkpoint molecules that block the PD-1/PD-L1 axis (i.e. pembrolizumab and nivolumab) or CTLA-4 (i.e. ipilimumab) stimulate antitumor immunity, through the reactivation of T-cell activities. Several immunotherapeutic approaches directed at avoiding tumor immune evasion are being investigated. They include T-cell checkpoint agonists or inhibitors for T-cell–activating pathways, cytokines IL-15 and IL-12, elimination of immunosuppressive cells and therapeutic vaccines.
Immunotherapy gains in complexity. Consequently, pathologists have a pivotal important role in the identification and quantification of cellular and molecular biomarkers in tissues and liquid biopsies reflecting not only the nature but also the degree of the antitumor immune response.
It has become clear that the predictive value of PD-L1 testing alone for immune checkpoint blockade in the selection of patients with genitourinary cancers is not enough to achieve positive results. The combination of PD-L1 expression with other new potential biomarkers for immunotherapy, like molecular subtyping, immune cell gene expression profiling and tumor mutational burden, is needed for an optimal personalized selection of patients as well as to predict response to immunotherapy.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewers’ Disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Junker K, Eckstein M, Fiorentino M, et al. PD1/PD-L1 axis in uro-oncology. Curr Drug Targets. 2020;21(13):1293–1300.
- Seliger B. Basis of PD1/PD-L1 therapies. J Clin Med. 2019;8(12):2168.
- Giunchi F, Gevaert T, Scarpelli M, et al. Status of programmed death ligand 1 (PD-L1) by immunohistochemistry and scoring algorithms. Curr Drug Targets. 2020;21(13):1286–1292.
- Gevaert T, Cimadamore A, Eckstein M, et al. Predictive biomarkers for immunotherapy in the treatment of advanced urothelial carcinoma: where we stand and where we go. Future Oncol. 2019;15(19):2199–2202.
- Suzman DL, Agrawal S, Ning YM, et al. FDA approval summary: atezolizumab or pembrolizumab for the treatment of patients with advanced urothelial carcinoma ineligible for cisplatin-containing chemotherapy. Oncologist. 2019;24(4):563–569.
- Eckstein M, Cimadamore A, Hartmann A, et al. PD-L1 assessment in urothelial carcinoma: a practical approach. Ann Transl Med. 2019;7(22):690.
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–322.
- Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197-218.
- Santoni M, Massari F, Cheng L, et al. PD-L1 inhibitors for the treatment of prostate cancer. Curr Drug Targets. 2020;21(15):1558–1565.
- Rizzo A, Mollica V, Cimadamore A, et al. Is there a role for immunotherapy in prostate cancer?. Cells. 2020;9(9):2051.
- Fay EK, Graff JN. Immunotherapy in prostate cancer. Cancers (Basel). 2020;12(7):1752.
- Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and treg skewing. Clin Cancer Res. 2008;14(11):3254–3261.
- Santoni M, Massari F, Di Nunno V, et al. Immunotherapy in renal cell carcinoma: latest evidence and clinical implications. Drugs Context. 2018;7:212528.
- Cimadamore A, Massari F, Santoni M, et al. PD1 and PD-L1 Inhibitors for the treatment of kidney cancer: the role of PD-L1 assay. Curr Drug Targets. 2020;21(16):1664–1671.
- Bakouny Z, Braun DA, Shukla SA, et al. Integrative molecular characterization of sarcomatoid and rhabdoid renal cell carcinoma. Nat Commun. 2021;12(1):808.
- Graham J, Wells C, Dudani S, et al. Effectiveness of first-line immune checkpoint inhibitors (ICI) in advanced non-clear cell renal cell carcinoma. J Clin Oncol. 2021;39(suppl 6):316.
- Cheng X, Dai H, Wan N, et al. Interaction of programmed death-1 and programmed death-1 ligand-1 contributes to testicular immune privilege. Transplantation. 2009;87(12):1778–1786.
- Fankhauser C, Curioni-Fontecedro A, Allmann V, et al. Frequent PD-L1 expression in testicular germ cell tumors. Br J Cancer. 2015;113(3):411–413.
- Cierna Z, Mego M, Miskovska V, et al. Prognostic value of programmed-death-1 receptor (PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann Oncol. 2016;27(2):300–305.
- Adra N, Einhorn LH, Althouse SK, et al. Phase II trial of pembrolizumab in patients with platinum refractory germ-cell tumors: a hoosier cancer research network study GU14-206. Ann Oncol. 2018;29(1):209–214.
- Necchi A, Bratslavsky G, Chung J, et al. Genomic features for therapeutic insights of chemotherapy-resistant, primary mediastinal nonseminomatous germ cell tumors and comparison with gonadal counterpart. Oncologist. 2019;24(4):e142–e5.
- Mego M, Svetlovska D, Chovanec M, et al. Phase II study of avelumab in multiple relapsed/refractory germ cell cancer. Invest New Drugs. 2019;37(4):748–754.
- Udager AM, Liu TY, Skala SL, et al. Frequent PD-L1 expression in primary and metastatic penile squamous cell carcinoma: potential opportunities for immunotherapeutic approaches. Ann Oncol. 2016;27(9):1706–1712.
- Santoni M, Heng DYC, Aurilio G, et al. Combining radiotherapy with immunocheckpoint inhibitors or CAR-T in renal cell carcinoma. Curr Drug Targets. 2020;21(4):416–423.
- Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11(10):702–711.
- Santoni M, Massari F, Aurilio G, et al. Designing novel immunocombinations in metastatic renal cell carcinoma. Immunotherapy. 2020;12(17):1257–1268.
- Pandha H, Pawelec G. Immune checkpoint targeting as anti-cancer immunotherapy: promises, questions, challenges and the need for predictive biomarkers at ASCO 2015. Cancer Immunol Immunother. 2015;64(9):1071–1074.
- Escudier B, Porta C, Schmidinger M, et al. ESMO guidelines committee. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(5):706–720.
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290.
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127.
- Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. PMID: 33657295 N Engl J Med. 2021;3849:829–841.
- Massari F, Mollica V, Rizzo A, et al. Safety evaluation of immune-based combinations in patients with advanced renal cell carcinoma: a systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19(10):1329–1338.
- Al Harthy M, Redman J, Madan RA. Novel immunotherapy combinations for genitourinary cancers. Exp Opin Biol Ther. 2020;20(3):253–262.
