Abstract
Malvella sherardiana (L.) Jaub & Spach (Malvaceae) is a perennial herbaceous plant listed as Vulnerable in the Red List of threatened plant species for Spain. The germination characteristics under controlled conditions of light and temperature of M. sherardiana seeds were studied. Mechanical scarification, cold stratification, liquid nitrogen, freezing, hot water, dry heat, sulphuric acid, soaking in distilled water and soaking in gibberellic acid were used as pre-sowing treatments applied for enhancing germination. The untreated seeds showed a high dormancy at all temperature regimens tested and sulphuric acid scarification drastically improved final germination percentage and germination rate. Germination of seeds soaked in concentrated sulphuric acid arithmetically increased as soaking time increased (from 5 min to 3 h), but seeds soaked in acid for more than 4 h failed to germinate. The application of a gibberellic acid solution (1000 mg l−1) increased the germination percentages for all tested soaking times in sulphuric acid. However, it was only significantly effective for the soaking time of 3 h. The other pre-sowing treatments resulted in some germination, but none resulted in greater germination than sulphuric acid. As embryos are fully developed and the seed coat is water permeable, we conclude that M. sherardiana seeds present physiological dormancy. The main conclusion of the study is that dormancy of M. sherardiana seeds is attributed to mechanical restriction of the embryo caused by the tough seed coat. This is the first report on germination requirements of the Malvella genus.
Introduction
Malvella is a small genus in the mallow family (Malvaceae). The Malvella genus includes four species, Malvella sherardiana (L.) Jaub & Spach being the only European species (Paiva and Nogueira Citation1993). The other three Malvella species are American species (www.malvaceae.info). Malvella sherardiana is a perennial herb that grows in ruderal areas and rain-fed crops and grasslands (González Talaván et al. Citation2004). This hemicryptophyte usually grows on dry soils, between 200 and 1000 m above sea level (Paiva and Nogueira Citation1993). The species is distributed throughout southwestern Asia and the eastern Mediterranean, where it exists, in clear disjunction, with a few known populations in central and southern Iberian Peninsula (Romero Martín Citation1996).
It is a highly threatened species in Spain with only a few known populations in Córdoba province (southern Spain), one population in Arévalo (Ávila province, central Spain) and another in Coslada (Madrid Community, central Spain) (Romero Martín Citation1996). The population located in Coslada, one of the largest Iberian populations of this species (over 300 individual plants) and only known location for the species in Madrid (Martínez Labarga Citation2013), was recently razed for building warehouses. Malvella sherardiana is listed as Vulnerable in the Red List of threatened plant species for Spain (González Talaván et al. Citation2004).
Propagation of M. sherardiana is by seed and, therefore, information on seed germination characteristics of the species is essential for increasing the success of conservation programmes for this threatened plant. The coat-imposed seed dormancy is due to either the impermeability of the coat to water and/or gases, the mechanical prevention of radicle extension, or the seed (or fruit) coat preventing inhibitory substances from leaving the embryo or by supplying inhibitors to the embryo (C. Baskin and J. Baskin Citation2014). In several Malvaceae species, physical dormancy (i.e. hard seed coat block of the germination process by not allowing the passage of water to the embryo) is the most important type of dormancy present in seeds (Egley, Paul, and Lax Citation1986; Van Assche and Vandelook Citation2006; Verma and Kasera Citation2006; El Balla, Saidahmed, and Makkawi Citation2011; C. Baskin and J. Baskin Citation2014). In addition to physical dormancy, other types of dormancy may be involved (Van Assche and Vandelook Citation2006). The Kew Gardens Seed Information Database (http://data.kew.org/sid/) is one of the most relevant data sources, reporting data for most genera of Malvaceae, but unfortunately there are no data for the Malvella genus. Therefore, to our knowledge this is the first study on germination characteristics of the Malvella genus.
The aim of the present study was to investigate the germination requirements of M. sherardiana. The specific objectives were: (1) to determine water uptake during seed imbibition, (2) to measure the seed coat mechanical resistance of M. sherardiana seeds, (3) to evaluate germination characteristics (final germination percentage and mean germination time) of seeds to different temperature regimens, and (4) to investigate the most appropriate pre-sowing treatments for enhancing germination.
Material and methods
Seed collection
Malvella sherardiana fruits are schizocarps composed of 8 to 15 mericarps. Each mericarp is inflated, single-seeded and indehiscent. Their reniform seeds (2–2.5 mm long) are dark brown, and densely warty (Paiva and Nogueira Citation1993). Ripe fruits, belonging to 20 different individuals chosen at random, were collected in July 2013 from a wild-growing population in the vicinity of the village of Coslada (40°25' N, 3°34' W, 620 m above sea level), eastern Madrid Community, central Spain. Then, seeds (Figure ) were manually extracted from dried mericarps in the laboratory, kept in paper bags, and stored dry under laboratory conditions (at about 23°°C, under darkness, 30–35% relative humidity) until the start of the trials in September 2013. Seeds with visible damage were excluded from the experiments.
Water uptake during seed imbibition
Water imbibition experiments were performed both with scarified and non-scarified seeds (intact seeds) for actual comparison. Seeds were scarified by soaking in concentrated sulphuric acid (H2SO4, 96%; three volumes of acid for each volume of seeds) for 3 h. Scarified seeds were immediately washed in running water to remove any trace of acid, and then stored at room temperature (~23°°C) for 24 h before being tested for water imbibition. To determine water uptake capacity during seed imbibition, three replications of 100 scarified and non-scarified seeds were weighed using an analytical balance with an accuracy of 0.01 mg and then placed in glass Petri dishes on two discs of filter paper moistened with distilled water at room temperature (~23°°C). After each imbibition period (from 0.5 to 48 h), seeds were taken out of the Petri dishes, quickly surface-dried with filter paper, reweighed and returned to the dishes. Percentage of water uptake (mean value ± standard error) was calculated as the amount of water taken up relative to initial seed mass.
Measurement of the seed coat resistance
Seed coat resistance may restrict embryo growth and therefore no germination occurs. A Texture Analyser (TA.XTplus, Stable Micro Systems Ltd., Godalming, Surrey, UK) was used to measure the mechanical resistance of the M. sherardiana seed coat. A 36-mm cylinder probe with radius (P/36R) using a 30 kg load cell was used. The compression test finished when the probe achieved a deformation of 0.7 mm at a speed of 0.1 mm s−1. The mechanical resistance was quantified through the force (N) needed to achieve the first fracture of the seed coat, obtained from the force–deformation curve. The resistance of seeds (n = 30) was measured after 0 (control), 1, 3, 5 and 7 h of soaking in concentrated H2SO4 at room temperature (~23°C).
General seed germination trials
Seeds were tested for germination at four different constant temperatures (10°C, 15°C, 20°C, 25°C) under a 16-h light photoperiod (light was provided by cool white fluorescent tubes with an irradiance of 35 μmol m2 s−1), and the alternate temperature regimens of 15/25°C, 20/30°C and 25/35°C (the highest temperature for 16 h in light and the lowest one for 8 h in dark). In all trials, four replicates of 25 seeds each were tested for germination on top of two sheets of filter paper (previously moistened with 3.5 ml distilled water) in 7-cm-diameter glass Petri dishes. Filter papers were rewetted regularly with distilled water as required. Dishes were checked three times a week over a total of a 13-week test period and germinated seeds were counted and removed. Seeds were considered germinated on emergence of the radicle from the seed coat (Figure ). In all germination trials, seeds that had not germinated at the end of the incubation period were opened to determine whether they were empty and whether they contained a firm white embryo.
Pre-sowing treatments
Nine pre-sowing treatments were applied in order to enhance seed germination:
| • | Mechanical scarification. Abrasion of seeds was carried out between two sheets of fine-grained sandpaper. | ||||
| • | Cold stratification. Seeds were cold stratified in moist vermiculite under darkness at 5°C for 16 weeks. | ||||
| • | Liquid nitrogen. Seeds were placed in plastic cryovials and directly immersed in liquid nitrogen (LN, –196°C) for 1 min. The subsequent warming procedure consisted of removing the samples from the cryogenic container and allowing them to warm under room temperature (~23°C) to equilibrium (24 h). | ||||
| • | Freezing and freeze-thawing. Seeds were stored in a freezer at –80°C for 24 h in plastic cryovials and then immediately treated with or without hot water at 90°C for 5 seconds. | ||||
| • | Soaking in distilled water. Seeds were soaked in distilled water (three volumes of water for each volume of seeds) at room temperature (~23°C) for 24 or 48 h. | ||||
| • | Hot water. Seeds were immersed in distilled water (three volumes of water for each volume of seeds) at 80°C for 5 or 30 min and at 100°C for 1, 5 and 30 min. | ||||
| • | Dry heat. Seeds, spread on glass dishes, were placed in an oven at 100°C for 5, 10, 30 and 60 min. | ||||
| • | Sulphuric acid. Concentrated H2SO4 (96%; three volumes of acid for each volume of seeds) was used to soak the seeds for different durations, between 5 min and 7 h (see Table ). Seeds were then immediately washed in running water to remove any trace of acid, before being tested for germination. | ||||
| • | Soaking in gibberellic acid. After each one of above treatments, a lot of 100 treated seeds (four replications of 25 seeds each) was directly sown and another lot of 100 treated seeds was soaked for 24 h at room temperature (~23°C) in an aqueous gibberellic acid solution (GA3, 1000 mg l−1; three volume of GA3 solution for each volume of seeds) before sowing. | ||||
Table 1. Final germination percentage and mean germination time (MGT, days) of Malvella sherardiana seeds after soaking in concentrated sulphuric acid for different times.
After all these treatments, pre-treated seeds were tested for germination at alternating temperatures of 15/25°C under a 16-h light photoperiod. Untreated seeds (with and without GA3) were sown in the same conditions and they were used as control seeds.
Data analysis
For all experiments, final germination percentage (mean value ± standard error) was calculated. The final germination percentages were arcsine transformed and then subjected to analysis of variance (ANOVA; untransformed data appear in the Tables) using the computing package SPSS (SPSS Inc., Chicago, IL, USA). The transformed data were checked for normality and homogeneity of variances before ANOVA. In all of the germination trials, the number of empty seeds in each replicate was always excluded when calculating the final germination percentage. Moreover, for seeds soaked in H2SO4, mean germination time (MGT, mean value in days ± standard error) was calculated. This parameter was determined according to the following formula: MGT = ΣDN/ΣN; where D is the number of days counted from the date of sowing and N is the number of seeds germinated on day D (Ellis and Roberts Citation1981). The value of MGT was not calculated when germination percentage was < 10%. Where ANOVA indicated a significant effect, a comparison of mean values was carried out through the least significant difference test at a 5% level of significance.
Results
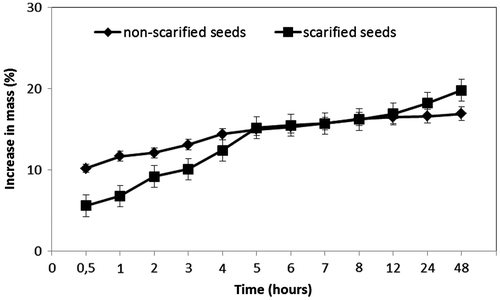
The mean fresh mass for a lot of 50 seeds was 303.55 ± 1.23 mg, corresponding to 6.07 ± 0.02 mg for single seeds. There were significant differences in mass of seeds before and after imbibition in water. Hence, M. sherardiana seeds imbibed water quickly and after 1 h of imbibition in distilled water the seed mass increase was 11.7 ± 0.16% for non-scarified seeds (intact seeds) and 6.8 ± 0.36% for seeds scarified with H2SO4 (Figure ). By 24 h, seed mass increase was 16.6 ± 0.23% and 18.2 ± 0.40%, respectively. After 48 h of soaking, seed mass increases were 16.9 ± 0.34% and 19.8 ± 0.80%, respectively. Therefore, both scarified and non-scarified seeds imbibe water in similar amounts. After 1 h of imbibition, non-scarified seeds reached 69% of the water uptake percentage reached after 48 h.
Figure 2. Mean (± SE) increase in mass of sulphuric-acid-scarified and non-scarified seeds of Malvella sherardiana incubated on filter paper moistened with distilled water at approximately 23°C.
None of the M. sherardiana seeds germinated for all tested temperature regimens (10°C, 15°C, 20°C, 25°C 20/30°C,°C 25/35°C), except for alternating temperatures of 15/25°C, where the final germination percentage was 2% and increased slightly to 5% after GA3 pre-treatment. The percentage of viable (firm embryos) seeds that did not germinate was > 95%.
Control seeds needed an average force of 58.8 N to fracture their coat. After soaking in H2SO4, the force needed was reduced slightly (e.g. 55.5 N for 7 h) but the ANOVA test was not significant (p > 0.05) for the effect of soaking time.
The final germination percentages of M. sherardiana seeds increased with increasing soaking time in H2SO4 for up to 3 h (Table ). However, after 3 h of soaking in H2SO4, germination declined gradually. Seed germination ranged from 5% to 77% for seeds subsequently soaked in GA3, and from 8% to 52% for seeds not soaked in GA3 (Table ). Both for soaked and non-soaked seeds in GA3, the highest germination percentages were reached by seeds soaked for 3 h in H2SO4 (77% and 54%, respectively) (Table ). Germination percentages reached by seeds soaked in GA3 were arithmetically higher than those of non-soaked seeds. However, these values were only significantly different (p < 0.01) for seeds previously soaked for 3 h in H2SO4. Both for untreated seeds and seeds treated with GA3, soaking in H2SO4 had a significant effect (p < 0.05) on germination in comparison to control (seeds untreated with H2SO4) for soaking times from 30 min to 5 h (Table ). Moreover, seedlings obtained from seeds scarified with H2SO4 were normal (i.e. seedlings with well-developed cotyledons, hypocotyl and radicle) in all the conditions used (Figure ).
Germination rate (as expressed by MGT) was significantly (p < 0.001) affected by soaking time in H2SO4 and soaking in GA3. Similarly, the interaction between both factors was significant (p = 0.004). The response to increasing times of soaking in H2SO4 for up to 3 h was a reduction in MGT both for soaked (from 31.8 to 4.1 days) and not soaked (from 48.6 to 9.4 days) seeds (soaked in GA3; Table ). For each time of soaking in H2SO4, significant differences were found between the MGT values reached by seeds that were soaked and not soaked in GA3 (Table ). Moreover, overall germination speed was higher for seeds soaked in a GA3 solution.
Mechanical scarification with sand paper significantly increased (p < 0.05) the final germination percentage when compared with control seeds (intact seeds) (28% versus 2%), whereas all other treatments (except for soaking in H2SO4) had no significant effects (p > 0.05) (Table ). However mechanical scarification was inefficient in increasing germination when followed with GA3 treatment (Table ). Moreover, significant differences (p < 0.001) between seeds that were soaked and not soaked in GA3 were only found for seeds subjected to dry heat for 10 min (Table ).
Table 2. Final germination percentage of Malvella sherardiana seeds after different pre-sowing treatments.
Discussion
Germination percentages achieved by M. sherardiana seeds were very low at all the tested incubation temperatures. Hence, only a small fraction (≤ 5%) could germinate without any pre-sowing treatment after 13 weeks of incubation at 15/25°C. The high viability of non-germinated seeds was also confirmed by the high germination percentage (77%) that was achieved by chemical scarification followed by GA3 application.
Seed coat hardness and impermeability to water are the most important causes of the low germination of several Malvaceae species (Egley, Paul, and Lax Citation1986; Torres, Long, and Zalba Citation2008; Verma and Kasera Citation2006; El Balla, Saidahmed, and Makkawi Citation2011; C. Baskin and J. Baskin Citation2014). Van Assche and Vandelook (Citation2006) found that seeds of three Malva species (Malva moschata L., Malva neglecta Wallr. and Malva sylvestris L.) have impermeable seed coats. However, not all members of this family have water-impermeable seeds (Baskin, Thompson, and Baskin Citation2006). Finch-Savage and Leubner-Metzger (Citation2006) reported seeds of the Malvaceae to be non-dormant, physically dormant or with a combinational dormancy (physical + physiological dormancy). Santo, Mattana, and Bachetta (Citation2015) reported the presence of combinational dormancy in Lavatera agrigentina Tineo seeds and only of physical dormancy in Lavatera pallescens Morris and Lavatera triloba. L. Malvella sherardiana intact seeds absorbed water quickly during the first 24 h of imbibition in distilled water. Besides, both scarified and non-scarified seeds imbibed similar amounts of water and significantly higher imbibition rates in scarified seeds were not found. Hence, the seed coat does not prevent absorption of water by M. sherardiana seeds. Therefore, these seeds did not exhibit physical dormancy, according to the classification system of J. Baskin and C. Baskin (Citation2004), where physical dormancy is defined as the result of a water-impermeable layer in the seed or fruit. As has been seen in several Malvaceae species (Van Assche and Vandelook Citation2006), M. sherardiana seeds have a well developed and differentiated embryo (i.e. cotyledons and radicle can be distinguished and no growth embryo was observed); therefore, they did not present morphological dormancy (as was established by J. Baskin and C. Baskin Citation2004). Besides, underdeveloped embryos are uncommon in the Malvaceae family (Forbis, Floyd, and Queiroz Citation2002; C. Baskin and J. Baskin Citation2014). As embryos are fully developed and seeds are water permeable, we conclude that M. sherardiana seeds have some type of physiological dormancy (see J. Baskin and C. Baskin Citation2004; C. Baskin and J. Baskin Citation2014).
The seed coat inhibited seed germination of M. sherardiana but it was not due to lack of imbibition of water by the seeds. The mechanical resistance test carried out has demonstrated the considerable toughness of the seed coat of M. sherardiana seeds. The force needed to fracture the seed coat of the species (approximately 59 N) is much higher than that for seeds of other species, e.g. 35 N for Onopordum nervosum Boiss. (Pérez-García and Pita Citation1989), 35 N for Helianthus annuus L. (Gupta and Das Citation2000), and < 1 N for Capsicum annuum L. (Watkins and Cantliffe Citation1983). However, H2SO4 scarification did not change significantly the force needed to fracture the tough seed coat of M. sherardiana. As H2SO4 scarification is highly effective in promoting seed germination of M. sherardiana, a possible effect on specific areas of seed coat could be suggested to justify the germination promotion by chemical scarification.
Chemical and mechanical scarification of the seed coat significantly increased seed germination, suggesting that the radicle is not able to overcome the mechanical resistance of the intact seed coat. Chemical and mechanical scarification reduces the mechanical restriction of the seed coat. Albumen can also participate in the mechanical resistance to radicle emergence. However in M. sherardiana seeds there is not well-developed albumen surrounding the embryo. Hence the radicle is able to emerge from the seed, although the growth potential of the embryo has not been increased (Baskin, Thompson, and Baskin Citation2006). Although M. sherardiana seeds possess a tough seed coat as revealed by scarification with H2SO4, the other tested pre-sowing treatments did not promote germination. Only mechanical scarification with sand paper significantly enhanced the final germination percentage compared with intact seeds, but much less than with H2SO4 scarification (28% versus 77%). Moreover, the germination of seeds scarified with H2SO4 was completely physiological.
According to C. Baskin and J. Baskin (Citation2014), there are three types of physiological dormancy: non-deep, intermediate, and deep. The duration of the cold stratification period required for breaking dormancy can indicate the type of physiological dormancy (J. Baskin and C. Baskin Citation2004). A 12-week period of cold stratification is adequate to break dormancy in seeds of many species (Baskin, Zackrisson, and Baskin Citation2002). In our study, the dormancy of M. sherardiana seeds was not broken by a long period of cold stratification (16 weeks). Moreover, as response of seeds to GA3 can be important in determining the type of physiological dormancy (J. Baskin and C. Baskin Citation2004), the effect of GA3 on germination of M. sherardiana was determined. The application of a GA3 solution (1000 mg l−1) increased the final germination percentages for all tested soaking times in H2SO4. However, it was only significantly effective for the soaking time of 3 h. This fact suggests that, although the main cause of dormancy of M. sherardiana seeds is the tough seed coat, GA3 application would help to overcome the mechanical resistance of the seed coat by increasing the growth potential of the embryo (according to J. Baskin and C. Baskin Citation2004; C. Baskin and J. Baskin, Citation2014). Moreover, scarification may increase sensitivity to GA3 (Baskin, Thompson, and Baskin Citation2006). As H2SO4 scarification is highly effective for promoting seed germination, M. sherardiana seeds would be classified into the non-deep physiological dormancy type (J. Baskin and C. Baskin Citation2004). Moreover, seedlings obtained from seeds soaked in GA3 were normal. However, and as occurs in others species (González-López and Casquero Citation2014), plants obtained from seeds soaked in GA3 showed a greater development of internodes than those from untreated seeds.
Our results show that the seed coat of M. sherardiana seeds plays an important role in seed dormancy by mechanically restricting embryo growth. Under natural conditions, only a reduced percentage (approximately 5%) of seeds is able to germinate; in contrast, most seeds (approximately 95%) did not germinate. Under natural conditions, a number of factors (including thermal oscillation between day and night, mechanical friction with soil particles, precipitation) would slowly and gradually alter the seed coat of M. sherardiana seeds, promoting germination in the field (Fenner and Thompson Citation2005). Hence, viable seeds of M. sherardiana may possess physiological dormancy due to mechanical restriction of the seed coat, which may form a persistent seed bank. Physiological dormancy is an adaptive trait because it allows seed germination over time and space (C. Baskin and J. Baskin Citation2014). Tough seed coat improves the survival of seeds in the soil, especially in adverse environmental conditions under any regimen of unpredictable disturbance because it can provide embryo protection (Kigel Citation1995; Fenner and Thompson Citation2005; C. Baskin and J. Baskin Citation2014; Martínez-Fernández, Martínez-García, and Pérez-García Citation2014).
In conclusion, our results suggest that the seed coat of M. sherardiana seeds is permeable to water but may mechanically interfere with the radicle emergence and, therefore, these seeds did not present physical dormancy. This fact should be highlighted because it is an important novelty in the Malvaceae family. Chemical scarification with H2SO4 was the technique that showed best results for improving seed germination, suggesting that dormancy in M. sherardiana seeds might be due to a tough seed coat. Our data provide useful information to develop conservation programmes of this threatened species, being the first report on the germination requirements of the Malvella genus.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Capes, Brazil [grant number BEX 10959-13-0].
Notes on contributors
Luciana Veiga-Barbosa (assistant professor) carried out the general seed germination trials and water uptake during seed imbibition. Her main interests are the ecophysiology of germination and seed dormancy of wild and cultivated species, in situ and ex situ plant conservation, plant genetic.
Carlos Ruiz (laboratory technician) collected the seeds and helped on the general seed germination trials. His main interests are the in situ and ex situ plant conservation, in vitro culture, plant taxonomy and morphology.
Eva C Correa (assistant professor) carried out the measurement of the seed coat resistance. Her main interests are the development and application of advanced modelling techniques in food and agriculture, multidimensional analysis, analytical modelling, optimal design, predictive control.
Félix Pérez-García (professor) collected the seeds, helped on general seed germination trials, made the analysis statistical and wrote the manuscript. His main interests are the ecophysiology of germination and seed dormancy of wild species, germination behaviour of endemic species, germination of species of arid and semi-arid areas, allelopathic effects, weed germination, fire effect on germination, germination of high mountain species, intra- and inter population variability in seed germination behaviour, seed viability and germination behaviour after preservation, in situ and ex situ plant conservation.
Acknowledgements
We thank Marta Huertas, Estrella Revenga and M. Elena González for your kind cooperation in the laboratory and collection seeds. Luciana Veiga-Barbosa was supported by the Capes, Brazil under Grant number BEX 10959-13-0.
References
- Baskin, C. C., and J. M. Baskin. 2014. “Seeds. Ecology, biogeography, and evolution of dormancy and germination.” San Diego, CA: Elsevier/Academic Press.
- Baskin, C. C., K. Thompson, and J. M. Baskin. 2006. “Mistakes in germination ecology and how to avoid them.” Seed Science Research 16: 165–168.
- Baskin, C. C., O. Zackrisson, and J. M. Baskin. 2002. “Role of warm stratification in promoting germination of seeds of Empetrum hermaphroditum (Empetraceae), a circumboreal species with a stony endocarp.” American Journal of Botany 89: 486–493.
- Baskin, J. M., and C. C. Baskin. 2004. “A classification system for seed dormancy.” Seed Science Research 14: 1–16.
- Egley, G. H., R. N. Paul, and A. R. Lax. 1986. “Seed coat imposed dormancy: Histochemistry of the region controlling onset of water entry into Sida spinosa seeds.” Physiologia Plantarum 67: 320–327.
- El Balla, M. M. A., A. I. Saidahmed, and M. Makkawi. 2011. “Effect of moisture content and maturity on hardseededness and germination in okra (Abelmoschus esculentus L. Moench.).” International Journal Plant Physiology and Biochemistry 3: 102–107.
- Ellis, R. H., and E. H. Roberts. 1981. “The quantification of ageing and survival in orthodox seeds.” Seed Science and Technology 9: 373–409.
- Fenner, M., and K. Thompson. 2005. The Ecology of Seeds. Cambidge, UK: Cambridge University Press.
- Finch-Savage, W. E., and G. Leubner-Metzger. 2006. “Seed dormancy and the control of germination.” New Phytologist 171: 501–523.
- Forbis, T. A., S. K. Floyd, and A. Queiroz. 2002. “The evolution of embryo size in Angiosperms and other seed plants: implications for the evolution of seed dormancy.” Evolution 56: 2112–2125.
- González-López, O., and P. A. Casquero. 2014. “Effects of GA3 pregerminative treatment on Gentiana lutea L. var. aurantiaca germination and seedlings morphology.” Hindawi Publishing Corporation. Scientific World Journal volume 2014, article ID 751279, 6 pages.
- González Talaván, A., L. Delgado Sánchez, S. Bernardos Hernández, and E. Rico Hernández. 2004. “Malvella sherardiana (L.) Jaub. & Spach.” In Atlas y Libro Rojo de la flora vascular amenazada de España [Atlas and Red Book of Threatened Vascular Flora of Spain], edited by Á. Bañares, G. Blanca, J. Güemes, J. C. Moreno, and S. Ortiz, 899. Madrid, Spain: Dirección General de Conservación de la Naturaleza.
- Gupta, R. K., and S. K. Das. 2000. “Fracture resistance of sunflower seed and kernel to compressive loading.” Journal of Food Engineering 46: 1–8.
- Kigel, J. 1995. “Seed germination in arid and semiarid regions.” In Seed development and germination, edited by J. Kigel and G. Galili, 645–699. New York, USA: Marcel Dekker.
- Martínez-Fernández, V., F. Martínez-García, and F. Pérez-García. 2014. “Census, reproductive biology, and germination of Astragalus gines-lopezii (Fabaceae), a narrow and endangered endemic species of SW Spain.” Turkish Journal of Botany 38: 686–695.
- Martínez Labarga, J. M. 2013. “La transformación de rural a urbano en el área metropolitana de Madrid, impacto en la conservación de la biodiversidad: Coslada, ejemplo de la falta de racionalidad en la planificación de los usos del suelo [The transformation from rural to urban in the metropolitan area of Madrid, impact on biodiversity conservation: Coslada, example of the lack of rationality in planning land use].” Paper presented at the VII International Seminar Biodiversity management and conservation, planning and management of agricultural and forestry resources. Gambarie d’Aspromonte, Italy, June 2–7.
- Paiva, J., and I. Nogueira. 1993. “Malvella Jaub. & Spach.” In Flora Iberica: plantas vasculares de la Península Ibérica e Islas Baleares [Flora Iberica : Vascular Plants of the Iberian Peninsula and Balearic Islands], edited by S. Castroviejo et al., Vol. 3, 208–209. Madrid, Spain: Real Jardín Botánico, CSIC.
- Pérez-García, F., and J. M. Pita. 1989. “Mechanical resistance of the seed coat during germination of Onopordum nervosum Boiss.” Seed Science and Technology 17: 277–282.
- Romero Martín, T. 1996. “Cuatro plantas poco conocidas de la Cuenca del Duero [Four poorly known plants from the Duero Basin].” Anales del Jardín Botánico de Madrid 54: 443–444.
- Santo, A., E. Mattana, and G. Bachetta. 2015. “Inter- and intra-specific variability in seed dormancy loss and germination requirements in the Lavatera triloba aggregate (Malvaceae).” Plant Ecology and Evolution 148: 100–110.
- Torres, Y. A., M. A. Long, and S. M. Zalba. 2008. “Reproducción de Pavonia cymbalaria (Malvaceae), una especie nativa con potencial ornamental [Reproduction of Pavonia cymbalaria (Malvaceae), a native species with ornamental potential].” Phyton 77: 151–160.
- Van Assche, J. A., and F. E. A. Vandelook. 2006. “Germination ecology of eleven species of Geraniaceae and Malvaceae, with special reference to the effects of drying seeds.” Seed Science Research 16: 283–290.
- Verma, V., and P. K. Kasera. 2006. “Effect of different seed and fertilizer treatments on growth and biomass of Sida cordifolia in semi-arid desert region of country.” Journal of Medical and Aromatic Plant Sciences 28: 23–26.
- Watkins, J. T., and D. J. Cantliffe. 1983. “Mechanical resistance of the seed coat and endosperm during germination of Capsicum annuum at low temperature.” Plant Physiology 74: 146–150.


