Abstract
Pluteus hispidulus, Pluteus kovalenkoi, Pluteus semibulbosus and Pluteus shikae of the family Pluteaceae (Basidiomycota: Agaricales) collected in the Aegean region of Turkey are new records for the Turkish mycota. In addition, this is the first report of these taxa growing in the forests under domination of Laurus nobilis and the relict endemic Liquidambar orientalis. Photographs, drawings, detailed descriptions and information about locality and habitat of the taxa are given.
Introduction
The genus Pluteus Fr. is the most widespread representative of the Pluteaceae Kotl. & Pouzar (Basidiomycota: Agaricales) with approximately 300 representatives in the world as a whole (Kirk et al. Citation2008). Pluteus species share such morphological characteristics as a basidiocarp with free lamellae independent of the stipe, a pink spore print, inamyloid basidiospore and inverse hymenophoral trama, and are a group of small or medium-sized agaric fungi that are generally not mycorrhizal (Vellinga and Schreurs Citation1985; Orton Citation1986; Singer Citation1986; Vellinga Citation1990). The most favoured habitats of these fungi are forests with old trees in the in humid regions. The Pluteus genus is reported to generally grow on the well-broken trunks or branches of trees (Orton Citation1986; Singer Citation1986; Vellinga Citation1990), and to occur on all continents except Antarctica (Singer Citation1986).
Recently, the genus Pluteus has been studied in different parts of the world (Justo et al. Citation2014; Menolli, de Meijer and Capelari Citation2015). In Turkey, 21 Pluteus taxa (P. atromarginatus, P. aurantiorugosus, P. brunneoradiatus, P. cervinus, P. cinereofuscus, P. dietrichii, P. ephebeus, P. leoninus, P. nanus, P. petasatus, P. phlebophorus, P. plautus, P. podospileus, P. pouzarianus, P. satur, P. robertii, P. romellii, P. roseipes, P. tomentosulus, P. thomsonii and P. umbrosus) have been recorded so far (Sesli and Denchev Citation2008; Solak et al. Citation2015). The hosts Fagus orientalis, Picea orientalis, Pinus brutia, Pinus nigra subsp. pallasiana, Populus alba, Populus nigra, Populus tremula, Quercus coccifera, Quercus ilex, Quercus pubescens and Salix alba are preferred by Pluteus taxa (Sesli and Denchev Citation2008; Solak et al. Citation2015). In the studies conducted on the islands of Greece, close to our study area, it was reported that P. atromarginatus, P. nanus, P. phlebophorus, P. plautus, P. pouzarianus, P. primus and P. salicinus were growing on the well-decayed wood of Abies cephalonica, Fagus sylvatica, Juniperus occidentalis, Platanus orientalis and Q. coccifera (Dimou, Zervakis and Polemis Citation2008; Polemis et al. Citation2012a). However, broad-leaved trees such as Laurus nobilis L., an evergreen plant endemic to the Mediterranean region (Davis, Mill and Tan Citation1988) growing on almost all of the Turkish coastlines, are the hosts of uncommon species such as Agaricus subrufescens, Fomitiporia punctata, Ganoderma adspersum, Ganoderma applanatum, Glomus deserticola, Glomus intraradices and Phellinus pseudopunctatus in Greece, Croatia and Italy (Kotlaba Citation1997; Hassiotis and Dina Citation2011; Simone and Annesi Citation2012; Polemis, Dimou and Zervakis Citation2013). In Turkey it was reported that Peziza punctispora, Fomitopsis palustris, Trametes versicolor, Chlorencoelia versiformis and Scotomyces subviolaceus grow in forests dominated by Liquidambar orientalis Mill. (Yilgor et al. Citation2013; Güngör, Allı and Işıloğlu Citation2013; Çolak et al. Citation2015).
In the present study, four Pluteus records (Pluteus hispidulus, Pluteus kovalenkoi, Pluteus semibulbosus and Pluteus shikae) are given as new taxa for the Turkish mycobiota. At the same time, their unusual habitats such as L. nobilis or relictual endemic L. orientalis, the latter species with a local distribution in the southwestern coastal area of Turkey (Köyceğiz, Fethiye, Marmaris and Milas) (Taşkın et al. Citation2007), are reported as hosts of Pluteus for the first time.
Materials and methods
Field work was undertaken in the provinces of Denizli, Muğla, Aydın, İzmir, Manisa, Uşak, Afyonkarahisar and Kütahya in the Aegean region of Turkey. Field studies were conducted mostly in the autumn and spring, the period during which the macrofungi produce fruiting bodies.
The macroscopic descriptions and images of the basidiomata of the taxa in the study were identified by observation of fresh or dried specimens. For microscopic analyses, the dried materials were rehydrated in 3% NaOH or 5% KOH and stained with Congo red. The following abbreviations are used in the descriptions: Lm for the average length of all the measured basidiospores, Wm for the average width of all the measured basidiospores, Q for the quotient of length and width of all the measured basidiospores, and Qm for the average of all calculated Q values for all basidiospores measured. At least 30 basidiospores from each basidioma were measured.
Identification of the taxa and descriptive terms for morphological and microscopic features were based on the following literature: Orton (Citation1986), Vellinga (Citation1990), Breitenbach and Kränzlin (Citation1995), Heilmann-Clausen (Citation2008), Justo et al. (Citation2014). After reviewing the samples using the latest studies of Sesli and Denchev (Citation2008) and Solak et al. (Citation2015), they were determined as being new records for the mycobiota of Turkey. Taxa, family and author citations are quoted according to Kirk et al. (Citation2008), Index Fungorum (Citation2016) and MycoBank (Citation2016). The taxa investigated in this study are deposited in the fungaria of the Pamukkale University Mushroom Research and Application Centre (PAUMMER).
Results
New records of Pluteus for Turkey
The distribution of the specimens of Pluteus found in the Aegean region of Turkey is given in Figure .
Figure 1. Distribution of new records of Pluteus in the Aegean region of Turkey according to the specimens examined. ■, Pluteus hispidulus, ●, Pluteus kovalenkoi, ▲, Pluteus semibulbosus and ★, Pluteus shikae.
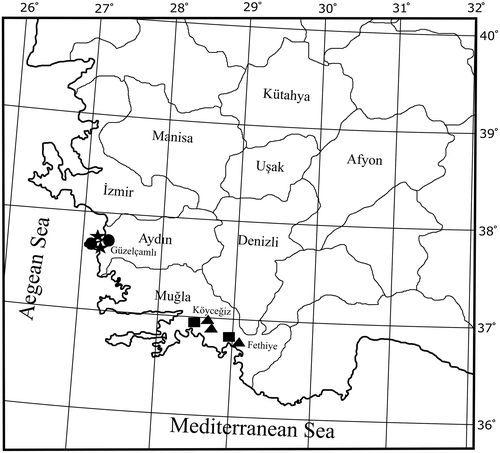
Pluteus hispidulus (Fr.) Gillet, Hyménomycètes (Alençon): 391 (1876). Figure .
Figure 2. Pluteus hispidulus. (a) Basidiomata. (b) Basidiospores. (c) Basidia. (d) Cheilocystidia. (e) Pleurocystidia. (f) Pileipellis elements. Scale bars 10 μm. All drawings from the specimen no. 275 (OKA) (photo and line drawings by O. Kaygusuz).
Pileus 8–15 mm across, convex to applanate with broad low umbo, straight, sometimes pellucid at margin, weakly hygrophanous, not translucently striate, arid, radially squamulose-fibrillose, distinctly hispid hairy, grey brown to dark brown, darker at centre, whitish at margin, scaly centre. Lamellae crowded, free, ventricose, thin, whitish or pale pink. Stipe 15–35 mm, central, cylindrical with slightly thickened base, fibrillose, solid, white to silvery, with white tomentum at base. Flesh of pileus and stipe very thin, smell indistinct, taste not recorded.
Basidiospores 5–8 × 4.5–6 μm, Lm × Wm = 6 × 4.5 μm, Q = 1.10–1.51 μm, Qm = 1.3 μm, broadly ellipsoid to ellipsoid, rarely subglobose, sometimes with granular content, smooth, thin-walled, hyaline. Basidia 23–35 × 7.5–9 μm, narrowly to broadly clavate, four sterigmata, without basal clamp. Cheilocystidia abundant, (20–)28–45(–55) × (8.5–)10–12(–18.5) μm, broadly clavate to fusiform, narrowly utriform, colourless, thin-walled. Lamella edge sterile. Pleurocystidia very scarce, (30–)35–40(–44) × 12–16 μm, broadly lageniform, subcapitate to fusiform, colourless, thin-walled. Pileipellis a differentiated cutis, consisting of elongate hyphal elements, with cylindrical terminal elements (40–)60–150(–190) × 9–12(–18) μm, with brown intracellular pigment, thick walled. Clamp connections not seen.
Habitat and phenology
Pluteus hispidulus grows singly or sometimes in small groups on the broken wood or logs buried in the ground of L. orientalis. It prefers hosts in soils that are rich in humus, are clayey-loamy, have a pH of 6.8–7.3, and are low in lime. It generally grows in shady places with damp ground, and has a long period of forming basidiomata (from early September to late November).
It is recorded from Muğla province (Köyceğiz distr.), in the southwest part of the Aegean region of Turkey; rare.
Specimens examined
Muğla province, Köyceğiz district, near Döğüşbelen town, L. orientalis forest, on rotted branches, 1 m above sea level, 20.09.2015, coll. and det. O. Kaygusuz 275 (OKA); Muğla province, Fethiye district, near Yanıklar town, L. orientalis forest, on rotted branches, 3 m above sea level, 16.11.2015, coll. and det. O. Kaygusuz 181 (OKA).
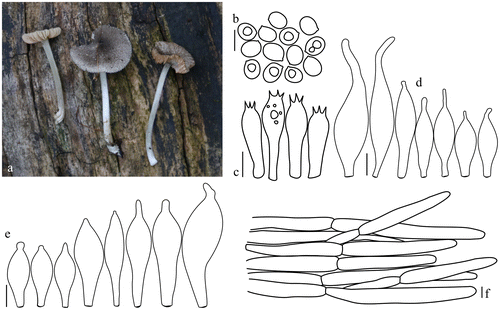
Pluteus kovalenkoi E.F. Malysheva, Phytotaxa 180(1): 50 (2014). Figure .
Figure 3. Pluteus kovalenkoi. (a, b) Basidiomata. (c) Basidiospores. (d) Basidia. (e) Pileipellis elements. (f) Pleurocystidia. (g) Cheilocystidia. Scale bars 10 μm. All drawings from the specimen no. 123 (OKA) (photo and line drawings by O. Kaygusuz).
Pileus 30–50 mm across, initially campanulate becoming applanate-convex with broad low umbo, surface smooth, with well-defined squamules at centre, whitish, brownish or smoky, translucently striate at margin, dry. Lamellae crowded, free, slightly ventricose, broad, at first whitish, later pink, concolorous edges. Stipe 65–78 × 4–6 mm, cylindrical, broad towards base, surface whitish or white-cream, solid. Contex in stipe and pileus white. Smell indistinct. Taste not recorded.
Basidiospore (6.4–)6.8–8.0(–9.1) × (4.3–)4.4–5.1(–5.3) μm, Lm × Wm = 7.3 × 4.7 μm, Q = (1.4–)1.45–1.7(–1.8) μm, Qm = 1.6 μm, broadly ellipsoid to oblong, smooth. Basidia 15–30 × 5–8 μm narrowly to broadly clavate, four sterigmata, without basal clamp. Pleurocystidia metuloid, numerous, 65–95 × 10–28 μm, variable in shape, mainly narrowly to broadly fusiform or broadly utriform, provided with 2–3(–4) apical hooks (generally entire, sometimes obtuse and poorly developed), hyaline, thick-wall. Lamellar edges sterile. Cheilocystidia 28–56 × 12–25 μm, clavate or broadly clavate, some spheropedunculate or utriform, hyaline, thin-walled, crowded. Pileipellis a cutis, with terminal elements 75–115 × 10–18 μm; individual elements mostly cylindirical, hyaline or with pale yellow-brown intracellular pigment, thin-walled. Stipitipellis a cutis, 10–18 μm wide hyphae, cylindrical, hyaline or with pale yellow-brown intracellular pigment, thin, with smooth walls. Clamp connections present, but not at every septum.
Habitat and phenology
Solitary or a few together, growing on well-decayed wood of L. nobilis. Pluteus kovalenkoi prefers host growing in soils that are rich in humus, clayey-loamy and with medium lime, and with a pH of 7.2–7.5. The specimens are generally found in north-facing, damp and rather dark habitats in piedmont areas (to 27 m above sea level) or on coastlines. The species has a long period (from early February to late April) of forming basidiomata.
It is recorded from Aydın province (Kuşadası distr.), in the southwest of the Aegean region of Turkey; rare.
Specimens examined
Aydın province, Kuşadası district, near Güzelçamlı town, on rotted pieces of L. nobilis wood, 27 m above sea level, 20.03.2015, coll. and det. O. Kaygusuz 123 (OKA); Aydın province, Kuşadası district, near Güzelçamlı town, on rotted pieces of L. nobilis wood, branches or parts of the tree, 22 m above sea level, 16.04.2015, coll. and det. O. Kaygusuz 180 (OKA).
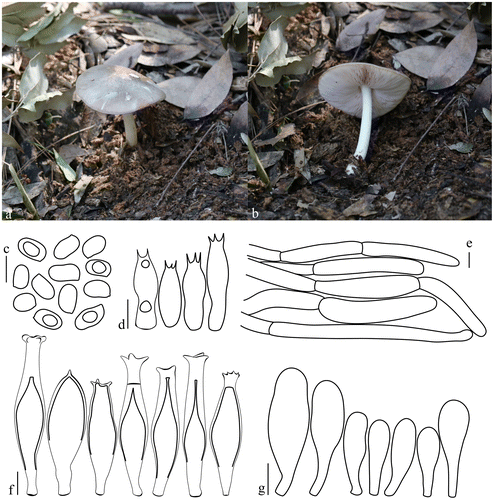
Pluteus semibulbosus (Lasch) Quél., Mém. Soc. Émul. Montbéliard 5: 543 (1875). Figure. .
Figure 4. Pluteus semibulbosus. (a, b) Basidiomata. (c) Basidiospores. (d) Basidia. (e) Pleurocystidia. (f) Pileipellis elements. (g) Cheilocystidia. Scale bars 10 μm. All drawings from the specimen no. 430 (OKA) (photo and line drawings by O. Kaygusuz).
Pileus 10–25 mm across, hemispherical or convex without any umbo, with straight margin, hygrophanous, translucently striate up to centre, dry, ochre or pale yellowish-brown at centre and almost white at margin, white tinged pale flesh-colour when moist, slightly granulose all over the surface. Lamellae crowded, free, ventricose, thin, white at first, later pink, with lamellulae. Stem 20–30 × 2–6 mm, central, cylindrical, with small basal bulb, surface almost white, smooth. Smell indistinct. Taste not recorded.
Basidiospores (5.6–)6.0–7.5(–7.7) × (4.7–)5.0–6.5(–6.8) μm, Lm × Wm = 6.7 × 5.6 μm, Q = 1.13–1.20 μm, Qm = 1.20 μm, broadly ellipsoid to subglobose, sometimes ovoid, with granular content, smooth, thin-walled. Basidia 25–35 × 7–10 μm, clavate, four sterigmata, without basal clamp. Pleurocystidia sparse, similar to cheilocystidia in shape, (28–)30–43(–45) × (8–)10–15(–17) μm, narrowly utriform to broadly lageniform with thin obtuse apex, narrow at base, hyaline, thin walled. Cheilocystidia numerous and variable in shape, (27–)31–45(–47) × (8–)9–16(–18) μm, mainly broadly clavate, utriform to fusiform with thin obtuse apex, narrow at base, hyaline, thin walled. Pileipellis a trichohymeniderm, 25–90 × 12–35 μm, broadly clavate to fusiform with terminal elements, thin-walled, with brown intracellular pigment. Clamp connections absent.
Habitat and phenology
Pluteus semibulbosus grows alone or sometimes in small groups on well-broken branches or logs of L. orientalis. It prefers hosts growing on soils that are rich in humus, clayey-loamy, low in lime, and which have a pH of 7.0–7.3. The specimens are generally found in habitats with damp ground and that are fairly dark, in thick forest or near the coast. The species has a long period of forming basidiomata, from September–November to February–April.
It is recorded from Muğla province (Köyceğiz & Fethiye distr.), in the southwest of the Aegean region of Turkey; sporadically.
Specimens examined
Muğla province, Köyceğiz district, near Döğüşbelen town, on well-broken branches of L. orientalis, 60 m above sea level 12.03.2015, coll. and det. O. Kaygusuz 146 (OKA); Muğla province, Köyceğiz district, Döğüşbelen town, on well-broken branches of L. orientalis, 60 m above sea level, 02.04.2015, coll. and det. O. Kaygusuz 149 (OKA); Muğla province, Fethiye district, near Yanıklar town on pieces of wood of L. orientalis, 9 m above sea level, 21.10.2015, coll. and det. O. Kaygusuz 388 (OKA).
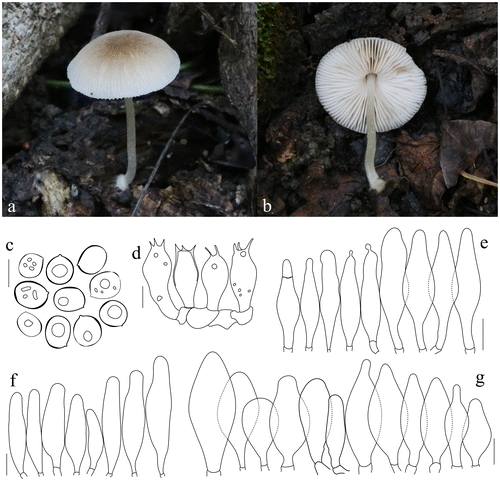
Pluteus shikae Justo & E.F. Malysheva, Phytotaxa 180(1): 48 (2014). Figure .
Figure 5. Pluteus shikae. (a, b) Basidiomata. (c) Basidiospores. (d) Basidia. (e) Pileipellis elements. (f) Pleurocystidia. (g) Cheilocystidia. Scale bars 10 μm. All drawings from the specimen no. 148 (OKA) (photo and line drawings by O. Kaygusuz).
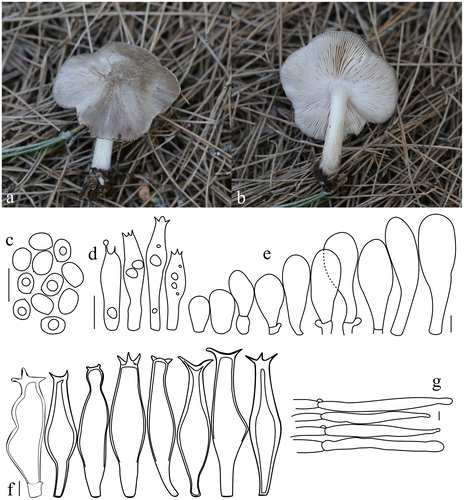
Pileus 15–40 mm across, initially hemispherical-campanulate to become broadly convex with low broad umbo, surface smooth and radially fibrillose, radially squamulose-fibrillose at centre, grey to dark grey-brown, broadly undulating pileal margin, dry, margin slightly translucent-striate. Lamellae crowded, broad, free, ventricose, at first white, later flesh-pink, flocculose edges. Stipe 25–55 × 3–5 mm, cylindrical, slightly swollen at the base, surface whitish, with greyish or grey-brown toned fibrils especially near the base. Ring absent. Context white in pileus and upper part of stipe, in some places slightly greyish. Odour and taste indistinct.
Basidiospores (5.9–)6.6–7.1(–7.9) × (4.3–)4.6–4.7(–5.0) μm, Lm × Wm = 6.9 × 4.7 μm, Q = 1.3–1.7 μm, Qm = 1.5 μm, ellipsoid to oblong, sometimes ovoid or slightly constricted in the middle, with granular content, smooth. Basidia 17–25 × 5–10 μm, clavate, four sterigmata, without basal clamp. Pleurocystidia metuloid, (55–)58–96(–100) × (12–)14–24(–27) μm, fusiform, narrowly fusiform to utriform with two to four apical hooks (generally entire, a few bifid), sometimes with small lateral hooks, hyaline, thick wall, abundant. Cheilocystidia (28–)33–58(–67) × (10–)13–22(–30) μm, clavate or narrowly clavate, more rarely narrowly utriform, hyaline, with brown intracellular pigment, thin-walled, crowded. Pileipellis a cutis, with terminal elements 94–158 × 6–15 μm, individual elements cylindrical, with brown intracellular pigment, smooth. Clamp connections present, but only in some septa.
Habitat and phenology
Solitary or a few together, growing on well-decayed wood of L. nobilis. Pluteus shikae prefers hosts growing on soils that are rich in humus, clayey, medium lime and with a pH of 7.2–7.3. They are generally found in habitats in mountainside areas that are north-facing, continuously damp and slightly dark, or on coastlines. The species has a short period of forming basidiomata, from early March to late April.
It is recorded from Aydın province (Kuşadası distr.), in the southwest of the Aegean region of Turkey; rare.
Specimens examined
Aydın province, Kuşadası district, near Güzelçamlı town, on well-broken logs of L. nobilis, 43 m above sea level, 28.03.2015, coll. and det. O. Kaygusuz 148 (OKA); Aydın province, Kuşadası district, Güzelçamlı town, on well-broken wood, branches or parts of the tree of L. nobilis, 81 m above sea level, 05.04.2015, coll. and det. O. Kaygusuz 164 (OKA); Aydın province, Kuşadası district, near Güzelçamlı town, on well-broken wood, branches or parts of the tree of L. nobilis, 55 m above sea level, 25.04.2015, coll. and det. O. Kaygusuz 183 (OKA).
Discussion
Pluteus hispidulus (Fr.) Gillet is recorded from European countries and Russian North Caucasus (Singer Citation1958; Orton Citation1986; Vellinga Citation1990; Malysheva and Svetasheva Citation2011), but had not previously been recorded with certainty in Turkey (Sesli and Denchev Citation2008; Solak et al. Citation2015). Pluteus hispidulus is similar to P. exiguus in the form of the cheilocystidia, but can be distinguished from the latter species by the structure of the pileipellis and shape of the hymenial cystidia. In addition, whereas the pileipellis structure of P. hispidulus is cutis with ascending elements, that of P. exiguus is a trichoderm. Pluteus hispidulus and P. exiguus can also be distinguished by habitat differences, because P. hispidulus has not been found growing in the soil (Orton Citation1986) in contrast to P. exiguus which is permanently growing in the soil (Kühner and Romagnesi Citation1956). In the present study, P. hispidulus was found in a new habitat, on the broken trunk of L. orientalis.
Pluteus kovalenkoi was first described from Russian Caucasus, Krasnodar Region (Justo et al. Citation2014); the fungus that was identified in our study is the first record in Turkey, and no records are known from other territories. Justo et al. (Citation2014) identified P. kovalenkoi with the molecular data and settled the confusion over its morphological similarity to the taxa P. shikae and P. brunneidiscus. Pluteus kovalenkoi is distinguished from P. shikae by the characteristics of the surface of the stipe, basidiospore size and the shape of the pleurocystidial hooks, and P. brunneidiscus differs by the shape of the pleurocystidia (Justo et al. Citation2014). Pluteus kovalenkoi is reported to grow on the well-decayed wood of conifers (Justo et al. Citation2014). In our study it was determined that this taxon grew on the well-decayed wood of L. nobilis, and that this is a new habitat for P. kovalenkoi. The ecological requirements of fungi can be an important character that can be used to show differences between taxa.
There is still no consensus about the identity of P. semibulbosus. Vellinga (Citation1990) consider it as a synonym of the variable taxon P. plautus. Other experts identify P. semibulbosus as a separate species (Heilmann-Clausen Citation2008; Malysheva and Svetasheva Citation2011). Pluteus semibulbosus can be distinguished from P. plautus by its pale pileus and in particular by its different habitat. It generally grows on the broken trunks of deciduous trees (Orton Citation1986; Vellinga Citation1990; Malysheva and Svetasheva Citation2011), whereas P. plautus grows on wood of dead hardwoods including conifers (Orton Citation1986; Breitenbach and Kränzlin Citation1995). Because of its cap colour and the bulb at the base of the white stipe, P. semibulbosus can be morphologically confused with P. boudieri and P. inquilinus. The pileipellis structure of P. semibulbosus is trichohymeniderm in contrast to that of P. boudieri having filamentous cuticle. Pluteus semibulbosus can be distinguished from P. inquilinus by the structure of the pileipellis and pleurocystidia shapes (narrowly utriform to broadly lageniform). Liquidambar orientalis appears to be a new host for P. semibulbosus.
Pluteus shikae has previously been reported in northern Japan (Hokkaido) and the Russian Far East (Primorsky Region), with no records between Far East and Asia Minor, and this is the first record discovered in Turkey (Aegean region). The molecular analysis revealed that P. shikae is a separate taxon from the morphologically similar P. brunneidiscus (Justo et al. Citation2014). It can be distinguished from P. brunneidiscus by relatively smaller dimensions and narrower basidiospores. Pluteus shikae has been reported to grow on the well-decayed wood of such trees as Quercus and Betula (Justo et al. Citation2014). It was determined in the present study that this taxon grew on well-decayed L. nobilis wood, and this is a new habitat for P. shikae. Hence, there is a need for further studies to show whether, as mentioned by Justo et al. (Citation2014), ecological requirements and geographical distribution have any importance in these Pluteus taxa.
The genus Pluteus is reported generally to grow on the well-broken trunks or branches of trees such as Abies, Acer, Alnus, Betula, Bignonia, Bursera, Castanopsis, Carex, Carya, Carpinus, Cocos, Eucalyptus, Fagus, Ficus, Fraxinus, Gleditsia, Lithocarpus, Nothofagus, Magnolia, Mangifera, Platanus, Picea, Pinus, Populus, Prunus, Pyrus, Quercus, Sequoia, Salix, Tilia, Tsuga and Ulmus (Pegler Citation1983; Orton Citation1986; Singer Citation1986, Citation1989; Vellinga Citation1990; Banerjee and Sundberg Citation1995; Horak Citation2008; Justo et al. Citation2014). In Greece, fungi of the Basidiomycota group have been recorded mostly on hosts such as Abies borisii-regis, A. cephalonica, Carpinus betulus, Castanea sativa, Cistus laurifolius, Cupressus sempervirens, Fagus sylvatica, F. orientalis, Juglans regia, Juniperus oxycedrus, Pinus brutia, P. halepensis, P. nigra, Picea abies, P. orientalis, Populus alba, P. nigra, Quercus coccifera, Q. conferta, Q. ilex, Q. pubescens and S. alba (Polemis et al., Citation2011, Citation2012a, Citation2012b). Laurus nobilis and Liquidambar orientalis are stated here for the first time as hosts of Pluteus representatives. In addition, the determination of whether the distribution of this genus is affected by ecological and geographical factors will continue in our later studies.
Conclusion
According to the current checklist and literature, 21 species of the genus Pluteus had been recorded in Turkey. Pluteus hispidulus, P. kovalenkoi, P. semibulbosus and P. shikae are recorded for the first time in Turkey, and the total number of Pluteus taxa has risen to 25 species. Liquidambar orientalis and L. nobilis are found as the new hosts for the Pluteus species discovered here.
Notes on contributors
Oğuzhan Kaygusuz is PhD student at Pamukkale University, Department of Biology. Contribution: designed the experiment, collected the samples and wrote the manuscript.
Kutret Gezer is associate professor at Pamukkale University, Department of Biology. Contribution: drafted and coordinated the project PAUBAP; he also checked and revised the draft of the manuscript.
Meryem Şeker is associate professor at Selçuk University, Department of Biology. Contribution: designed the experiment and contributed to the writing of the manuscript.
Acknowledgements
The authors would like to acknowledge the financial support of the Pamukkale University Scientific Research Projects Coordination Unit (PAUBAP) (project number: 2014FBE044).
References
- Banerjee, P., and W. J. Sundberg. 1995. “The genus Pluteus section Pluteus (Pluteaceae, Agaricales) in the midwestern United States.” Mycotaxon 53: 189–246.
- Breitenbach, J., and F. Kränzlin. 1995. Fungi of Switzerland. Vol. 4. Boletes and Agarics (2nd part). Verlag Mykologia: Switzerland.
- Çolak, Ö. F., I. Şen, N. Alkan, and M. Işıloğlu. 2015. “A new and interesting Peziza record from sweet gum forest in Turkey.” The Journal of Fungus 6 (1): 10–12.
- Davis, P. H., R. R. Mill., and K. Tan. 1988. Flora of Turkey and the East Aegean Islands. vol. 2. Edinburgh: Edinburgh University Press.
- Dimou, D. M., G. I. Zervakis, and E. Polemis. 2008. “Mycodiversity studies in selected ecosystems of Greece: IV. Macrofungi from Abies cephalonica forests and from other intermixed tree species (Oxya Mt., central Greece).” Mycotaxon 104: 39–42.
- Güngör, H., H. Allı, and M. Işıloğlu. 2013. “Three new macrofungi records for Turkey.” Turkish Journal of Botany 37: 411–413.
- Hassiotis, C. N., and E. I. Dina. 2011. “The effects of laurel (Laurus nobilis L.) on development of two mycorrhizal fungi.” International Biodeterioration and Biodegradation 65: 628–634.
- Heilmann-Clausen J. 2008. “Pluteus Fr.” In Funga Nordica. Agaricoid, boletoid and cyphelloid genera, edited by H. Knudsen and J. Vesterholt, 535–538. Copenhagen: Nordsvamp.
- Horak, E. 2008. Agaricales of New Zealand. Pluteaceae-Entolomataceae, Vol. 1, 1–305, Hong Kong: Fungal Diversity Press.
- Index Fungorum. 2016. Accessed 28 February 2016; www.indexfungorum.org
- Justo, A., E. F. Malysheva, T. M. Bulyonkova, E. C. Vellinga, G. Cobian, N. Nguyen, A. M. Minnis, and D. S. Hibbett. 2014. “Molecular phylogeny and phylogeography of Holarctic species of Pluteus section Pluteus (Agaricales: Pluteaceae), with description of twelve new species.” Phytotaxa 180: 1–85.
- Kirk, P., P. F. Cannon, D. W. Minter, and J. A. Stalpers. 2008. Ainsworth and Bisby’s Dictionary of the Fungi. 10th ed. Wallingford: CAB International.
- Kotlaba, F. 1997. “Some uncommon or rare polypores (Polyporales s.l.) collected on uncommon hosts.” Czech Mycology 50 (2): 133–142.
- Kühner, R., and H. Romagnesi. 1956. “Compléments à la flore analytique. VIII. Espèces nouvelles, critiques ou rares de Volvariacées.” Bulletin Trimestriel de la Société Mycologique de France 72: 181–249.
- Malysheva, E. F., and T. Y. Svetasheva. 2011. “Fungi non delineati.” Rare and noteworthy species of agarics from the Western Caucasus. Pars LXI, 1–104. Alassio: Edizioni Candusso.
- Menolli, J. N., A. A. R. de Meijer, and M. Capelari. 2015. “The genus Pluteus (Pluteaceae, Agaricales) from the State of Paraná, Brazil.” Nova Hedwigia 100: 101–157.
- MycoBank. 2016. Fungal Databases, Nomenclature and Species Banks, Accessed 28 February 2016; www.mycobank.org.
- Orton, P. D. 1986. “British Fungus Flora.” Agarics and Boleti 4: Pluteaceae: Pluteus and Volvariella. Edinburgh: Royal Botanic Garden.
- Pegler, D. N. 1983. “Agaric Flora of the Lesser Antilles.” Kew Bulletin (Additional Series), 9, 1–688, London: Stationery Office.
- Polemis, E., D. M. Dimou, and G. I. Zervakis. 2013. “The family Hymenochaetaceae (Agaricomycetes, Basidiomycota) in the islands of the Aegean Archipelago (Greece).” Plant Biosystems 147 (2): 306–314.
- Polemis, E., D. M. Dimou, D. Tzanoudakis, and G. I. Zervakis. 2012a. “Annotated checklist of Basidiomycota (subclass Agaricomycetidae) from the islands of Naxos and Amorgos (Cyclades, Greece).” Annales Botanici Fennici 49: 145–161.
- Polemis, E., D. M. Dimou, D. Tzanoudakis, and G. I. Zervakis. 2012b. “Diversity of Basidiomycota (subclass Agaricomycetidae) in the island of Andros (Cyclades, Greece).” Nova Hedwigia 95: 25–58.
- Polemis, E., D. M. Dimou, L. Pountzas, D. Tzanoudakis, and G. I. Zervakis. 2011. “Mycodiversity studies in selected ecosystems of Greece: 5. Basidiomycetes from woods dominated by Castanea sativa (Nafpactia Mts., Central Greece).” Mycotaxon 115: 535.
- Sesli, E., and C. W. Denchev. 2008. “Checklist of the myxomycetes, larger ascomycetes, and larger basidiomycetes in Turkey.” Mycotaxon 106: 65–67.
- Simone, D. D., and T. Annesi. 2012. “Occurrence of Ganoderma adspersum on Pinus pinea.” Phytopathologia Mediterranea 51 (2): 374–382.
- Singer, R. 1958. “Monographs of South American basidiomycetes, especially those of the east slope of the Andes and Brazil: 1. The genus Pluteus in South America.” Lloydia 21: 195–299.
- Singer, R. 1986. The Agaricales in modern taxonomy. 4th ed. Koenigstein: Koeltz Scientific Books.
- Singer, R. 1989. “New taxa and new combinations of Agaricales (Diagnoses Fungorum Novorum Agaricalium IV).” Fieldiana: Botany, New Series, 2, 1–133. Chicago: Field Museum of Natural History.
- Solak, M. H., M. Işıloğlu, E. Kalmış, and H. Allı. 2015. Macrofungi of Turkey. İzmir: University Press.
- Taşkın, B. G., V. Taşkın, K. Küçükakyüz, Ö. Varol, B. Çöl, and T. Arslan. 2007. “Esterase polymorphisms in relict endemic Liquidambar orientalis Mill. var. orientalis and L. orientalis Mill. var. integriloba Fiori populations in Turkey.” Journal of Cell and Molecular Biology 6: 137–146.
- Vellinga, E. C. 1990. “Pluteus Fr.” In Flora Agaricina Neerlandica, Vol 2, edited by C. Bas, Th.W. Kuyper, M.E. Noordeloos, and E.C. Vellinga, 31–55. Rotterdam: A.A. Balkema.
- Vellinga, E. C., and J. Schreurs. 1985. “Notulae ad floram agaricinam Neerlandicam−VIII. Pluteus Fr. in West Europe.” Persoonia 12: 337–373.
- Yilgor, N., D. Dogu, R. Moore, E. Terzi, and S. N. Kartal. 2013. “Evaluation of fungal deterioration in Liquidambar orientalis Mill. heartwood by FT-IR and light microscopy.” Bioresources 8 (2): 2805–2826.
