Abstract
The study of higher plant growth in space started in the 1950s both on the Russian and the American sides. The first experiments were intended to assess whether plants could grow outside Earth and to determine what differences there were between spaceflight-grown and Earth-grown plants. As plant-growth hardware started to adapt to spaceflight, more opportunities for plant experiments in space became available. Direct microgravity effects started being differentiated from confinement effects and Earth orbit started to become a laboratory where plants could be grown without the influence of Earth gravity. Experiments have shown that, with adapted ventilation, plant growth in space is similar to plant growth on Earth in 1 g, except for some morphological traits. However, only small-scale experiments on plant growth were performed in Earth orbit, which provided insufficient data on crop yield for reduced gravity environments. Challenges remain to grow plants in space, in terms of nutrient delivery, lighting and ventilation, but also on the choice of plant species and traits to favour. Additionally, significant effort must be made on mechanistic modelling of plant growth to reach a more thorough understanding of the intricate and combined physical, biochemical, and morphological phenomena involved, which is necessary to accurately control and predict plant growth in life-support systems. This review intends to list the main spaceflight effects to take into account for plant growth in space, as well as to give an overview of the current state of plant-growth hardware while stressing the challenges associated with their development.
Introduction
Growing plants in space requires a deep understanding of plant growth mechanisms (Hezard et al. Citation2010) and proficient know-how in controlled-environment agriculture (Steinberg, Ming and Henninger Citation2002; Kiss et al. Citation2014). For many decades, healthy plants have grown on spacecraft environments; Porterfield et al. (Citation2003) gave an extensive review of the evolution of plant growth experiments in space and associate hardware in the years 1960–2000. However, long-term effects of the space environment on plant growth and reproduction are not yet well known and understood, and could impact the role of plants as food source in bioregenerative life-support systems (Wolff et al. Citation2014). Indeed plants will play a critical role in the survival of human beings outside Earth for long-durational missions within the Solar System.
Environmental control and life-support systems
Survival of astronauts in space is ensured by Life-Support Systems (LSS), which have the functions of recycling water and revitalization of the atmosphere. On the International Space Station (ISS) the current standard are physicochemical LSS: oxygen is produced using water electrolysis in the Oxygen Generation Assembly and a Sabatier reactor uses the metabolically generated CO2 and the by-product of water electrolysis H2 to produce water (Bagdigian and Cloud Citation2005; Burkey et al. Citation2010); urine is recycled into potable water in the Urine Processor Assembly and water vapour is also recovered from the cabin air to be used as potable water (Carter Citation2010). Although these LSS are easier to set up and to control than bioregenerative LSS, they are unable to provide the astronauts with food. Only biological processes have the ability to grow fresh food. Bioregenerative LSS are based on living organisms (micro-organisms, algae, plants, fish, etc.) and therefore require very precise control to be efficient and reliable.
Research on bioregenerative LSS has been ongoing since the early 1950s (Salisbury, Gitelson and Lisovsky Citation1997; Wheeler Citation2010). BIOS 1 to 3 were the first bioregenerative LSS tested in the former USSR from 1965 to 1985, which included alga Chlorella vulgaris, plants and human subjects (Gitelson et al. Citation1989). The longest mission lasted for 180 days (half a year), consisting of three crewmates, performing air regeneration, water recycling and provision of vegetarian food (including wheat, chufa, beet, carrots), supplemented by dried meat, although urine and faeces were not recycled but simply stored (Gitelson and Lisovsky Citation2008). The BIOS systems demonstrated that, even on the longest test (6 months), the microbial population within the system could not be stabilized; there were also problems with system leakage and the building up of toxic oxides in the system atmosphere due to the incineration of inedible parts of the plants (Rygalov, Kovrov and Denisov Citation1997). In the 1980s, the NASA Controlled Ecological Life Support Systems program investigated food production and processing, nutrition, ecology of closed systems, and waste processing (Wheeler Citation2010). In the late 1980s, scale-up tests of the fundamental findings from the university laboratories were performed in the Biomass Production Chamber at the Kennedy Space Center. With an arable area of 20 m2, this large, atmospherically closed chamber was used to demonstrate food production, water recycling and atmospheric control, as well as testing the effects of a tightly closed atmosphere on plant growth. Tests performed in the Biomass Production Chamber provided baseline values for different candidate plant species, in terms of productivity, gas exchanges, evapotranspiration and mineral nutrition, which could be used in bioregenerative LSS calculations, as well as lessons learned for future similar studies (e.g. the necessity to emphasize the use of dwarf plants in space or the criticality to control volatile organic compounds to a low level in confined environments, since they negatively affect whole-plant growth and therefore also harvesting index) (Wheeler et al. Citation1996).
On the European side, the leading project for the last 26 years on regenerative life-support research has been the Micro-Ecological Life-Support System Alternative (MELiSSA) project led by the European Space Agency (ESA). Inspired by a lake ecosystem, the concept of MELiSSA is a closed loop organized in five compartments: three of which are based on micro-organisms degrading and transforming the organic wastes of the crew into elements that are used, together with carbon dioxide from the crew and organic wastes of the mission, to feed the fourth compartment – which is based on higher plants and algae, that in return provides food, oxygen and water to the fifth compartment, which is the crew (Gòdia et al. Citation2002; Lasseur et al. Citation2010). One challenge of the project is to avoid contamination between the different compartments, while ensuring a mass and energy balance of the loop. It was shown that about 90% of the water absorbed by plant roots is transpired, so entering the gas loop, and 10% is used for biomass creation in plants (Hezard et al. Citation2012). When plants only provide 20% of the astronauts’ diet, water recycling is achieved (Eckart Citation1994). Atmosphere can be recycled and all breathable oxygen provided when 50% of the astronauts’ diet is covered by plants (Eckart Citation1994). The average respiratory quotient of one human being is in the range of 0.8–0.9 (Poughon Citation1997), so the objective is to recycle 1.1 to 1.3 mol of oxygen per mol of carbon dioxide, which is achieved by all candidate crops of the MELiSSA project (e.g. wheat, rice, soybean, potato, lettuce) (Poughon Citation1997). The average harvest index of these crops is 50%, meaning that half of the biomass produced in the MELiSSA loop is edible and goes to the crew compartment, whereas the other half is inedible waste needing to be recycled (see Figure , where the mass fluxes to and from a higher-plant chamber are represented, including the crew compartment and a partial waste treatment). To have a reliable system, it is necessary to accurately predict behaviour of biological processes and control their activity at a compartment level and at a loop level. This is achievable by understanding the mechanisms, which govern such biological processes (see Figure ).
Figure 1. Diagram showing the different mass fluxes between the higher plant chamber (HPC) and the crew and waste treatment unit of a regenerative life-support system (not including crew organic waste treatment in this figure).
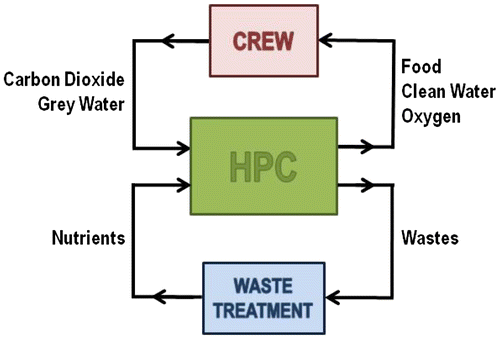
Figure 2. Diagram showing the links between a system, the experiments conducted on it, its mechanisms, and a control loop, which are the principles of mechanistic modelling. Information about the system enables us to understand mechanisms about this system, which provides knowledge on how to control it.
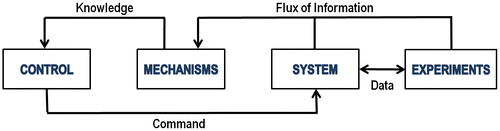
The MELiSSA approach is therefore to develop mechanistic models of the entire loop and in parallel to build and test lab-scale prototypes of each compartment. All three microbial compartments and the algae compartment are effectively modelled mechanistically (Cornet, Dussap and Gros Citation1998; Cogne, Gros and Dussap Citation2003; Ordoñez et al. Citation2004), while modelling efforts on the higher plant compartment are currently ongoing (Hezard et al. Citation2010, Citation2012). The main difference with existing agronomy models is that plants in bioregenerative LSS will grow in a confined environment, so slight variations in environmental parameters can strongly affect the whole system, because there are no buffer effects of the atmosphere, oceans or soil. Therefore it is critical to understand plant growth mechanisms, as well as variations of the physical environmental surrounding plants, using platforms, such as the ISS in Low Earth Orbit, which offers microgravity environments.
Understanding mechanisms of plant growth and development
The goal of many fundamental plant growth experiments in Earth orbit since the 1960s (Porterfield et al. Citation2003) has been to study plant growth and development, from germination to reproduction mechanisms, tropisms and circumnutation, as well as biochemistry and molecular biology (Paul et al. Citation2013). Experimenting in Earth orbit (space stations such as Mir or the ISS, Space Shuttle, or satellites), in parabolic flights, in sounding rockets, or even on clinostats, enables us to investigate certain growth mechanisms outside the influence of gravity. Phototropism and gravitropism have therefore been extensively studied in orbit and there has been a significant effort made to separate the overlapping effects of light and gravity on plant growth (Vandenbrink et al. Citation2014).
It was shown that the gaseous environment was very important for plant growth and development in space. It was initially thought that microgravity led to smaller plants and that their reproduction was impaired (Musgrave et al. Citation2005; Paul et al. Citation2013) but as plant growth hardware for space improved, these artefacts disappeared and it was shown that plants can grow normally in microgravity provided that the plants grow in a well-ventilated area (Monje, Stutte and Chapman Citation2005). A major result in space biology was the demonstration of a seed-to-seed growth of Arabidopsis thaliana on orbit during STS-68 mission, which showed no difference with ground controls (Kuang, Xiao and Musgrave Citation1996; Paul et al. Citation2013).
After reviewing the different effects of spaceflight on plant growth, which are critical for food production in space, this paper intends to provide an overview of state of the art plant-growth technologies developed for Earth orbit and planetary surfaces, while highlighting the remaining challenges of space agriculture.
Effects of spaceflight on plant growth
In early spaceflight experiments on plant growth and development, many observed effects that were attributed to microgravity were actually due to indirect spaceflight effects (Paul et al. Citation2013), such as confinement – leading to build-up of volatile organic compounds, such as ethylene – and lack of convection – resulting in super-elevated CO2 levels in the spacecraft and locally at plant-leaf surfaces (Monje et al. Citation2003). Elevated radiation levels also have negative, not yet fully known, effects on plant growth and development. On-orbit plant experiments can therefore be confounded by these spacecraft-specific artefacts (Musgrave Citation2002).
With the development of improved hardware for microgravity experiments minimizing indirect spaceflight effects, results on plant growth in space became more reliable and on-orbit plant growth appeared less chaotic than what was initially thought (Paul et al. Citation2013). It also seems that microgravity response is species- and cultivar-dependent, making it hard to find general patterns of plant growth and development in microgravity (Paul et al. Citation2013). The effects of microgravity on plant growth and development have been thoroughly reviewed in recent years (Wolverton and Kiss Citation2009; De Micco et al. Citation2014; Paradiso et al. Citation2014; Wolff et al. Citation2014; Kittang Jost, Hoson and Iversen Citation2015; Vandenbrink and Kiss Citation2016) and hence this review focuses on the aspects that are critical for plant cultivation in space. Radiation and magnetic field are also known to affect plant growth. As reviewed in Arena et al. (Citation2014), plants can sustain radiation doses one hundred times higher than mammals can and low radiation doses might lead to positive outcomes such as increase in growth and photosynthesis. Their effects are thoroughly reviewed in De Micco et al. (Citation2011) and Wolff et al. (Citation2014) but will not be discussed herein.
Cellular and biochemical scale
Secondary metabolism
Although it was shown that, provided with adequate ventilation in space, plant development is similar to that on Earth (Monje, Stutte and Chapman Citation2005), secondary metabolism is affected by altered hypo- and hyper-gravity as reviewed by Tuominen, Levine and Musgrave (Citation2009) and major changes in storage reserves were observed in the spaceflight environment, with seeds produced on orbit having different composition and developmental stages than seeds grown not only on Earth (Musgrave et al. Citation2005), but also on clinostats (Brown, Piastuch and Knott Citation1994). These changes in starch storage and metabolite production could impact the vigour and nutritional content of seeds produced in space plants (Musgrave, Kuang and Matthews Citation1997) and might affect the flavour of plants produced in space (Musgrave et al. Citation2005), which could become a problem on long-duration space missions where crews would rely on plant-based diets. De Micco and colleagues in 2006 and 2008 found that the amount and repartition of starch and other metabolites were different in soy seedlings grown on clinostat and on the ISS than in their 1 g control plants (De Micco, Aronne and De Pascale Citation2006; De Micco and Aronne Citation2008). The seed-to-seed cycle of on-orbit grown plants, especially seedling development and establishment, as well as gametogenesis and pollination, were thoroughly reviewed by De Micco et al. in Citation2014. They concluded that these responses to altered gravity conditions could be interpreted as stress-induced morphogenic responses (De Micco et al. Citation2014).
Cell growth and proliferation
Cell growth and proliferation are two related processes that are coupled in 1 g but they seem to be decoupled in microgravity, which could have an impact on plant growth and development and hence on food production in reduced gravity environments (Medina et al. Citation2015). Manzano et al. in Citation2009 found that cell proliferation was enhanced, whereas cell growth was decreased in microgravity; they hypothesized that this decoupling was due to acceleration in cell division caused by a shorter G2 phase, leading to the formation of more cells with shorter sizes (Manzano et al. Citation2009). This result was confirmed in 2010 by Medina et al., who found that meristematic cell proliferation was enhanced while meristematic cell growth was reduced in microgravity (Medina et al. Citation2010). This was reviewed by Herranz and Medina in Citation2014, who hypothesized that changes in cell growth and proliferation in non-specialized cells in microgravity could be a result of gravity resistance mechanisms (Herranz and Medina Citation2014).
Gene expression
Recent progress in gene expression studies (e.g. microarray technology) has enabled the identification of changes in gene expression in microgravity, as reported in a review by Wolverton and Kiss (Citation2009). Important results about gene expression in microgravity are (i) the over-expression of heat-shock-related genes, although plants were not exposed to elevated temperatures (Paul et al. Citation2005); (ii) the differential expression (down-regulation and up-regulation) of some genes in fern spores between 1 g and microgravity (Salmi and Roux Citation2008); (iii) the down-regulation of genes essential for normal root hair development (Known et al. Citation2015); (iv) a significant increase of proteins related to stress responses, defence and metabolism and a significant decrease of proteins related to auxin metabolism and trafficking (Mazars et al. Citation2014; Zhang et al. Citation2015). Herranz and Medina (Citation2014) hypothesized that microgravity is perceived by the plant transcriptome as a novel stressful environment and that the genome lacks adequate genes to respond accordingly; hence the observation of up- and down-regulation of certain genes in microgravity (Herranz and Medina Citation2014). Understanding mechanisms of gene expression in microgravity will enable the selection of plants that adapt the best to reduced-gravity environments and to genetically engineer plants that are adapted for spaceflight and planetary surfaces food production.
Indirect effects at the organ scale
Although the activity of photosystems and the expression of some proteins involved in photosynthesis can be affected by spaceflight (Giardi et al. Citation2013), photosynthesis rate does not seem to be affected by microgravity (Monje, Stutte and Chapman Citation2005; Wolverton and Kiss Citation2009). Changes observed at the organ scale are mostly indirect consequences of the lack of gravity, altering fluids behaviour (no buoyancy-driven convection) and dominating forces (surface tension is prevalent in microgravity). The influence of spaceflight environment and the interaction between gas exchange, photosynthesis, and plant nutrition were thoroughly reviewed by Wolff et al. (Citation2014), so a brief overview is given herein, highlighting how it could impact the growth of plants in space in future long-duration missions.
Domination of surface-tension forces
In microgravity, water creates thicker boundary layers around plant roots, which become oxygen deficient because of the roots’ respiration, leading to root hypoxia (Porterfield et al. Citation1997; Monje et al. Citation2003). Therefore it was often observed in the early on-orbit plant experiments that the plants showed hypoxia-like ultra-structural changes due to oxygen limitation (Slocum, Gaynor and Galston Citation1984; Monje et al. Citation2003). Hence it became necessary to develop microgravity-specific watering systems enabling water and nutrient delivery and roots aeration.
Lack of convection
The fact that there is almost no natural convection in microgravity has direct consequences on plant growth. Indeed without adequate ventilation, thicker mass boundary layers form around plant leaves, increasing the transport resistance by diffusion, which in turns reduces gas exchange at the leaf surface and around the roots, inducing a depletion of certain gases (e.g. O2, CO2) in these layers because of the plant’s activity such as respiration or photosynthesis, which can cause hypoxia (Porterfield Citation2002). This leads to a decrease in photosynthesis rates as reported during a parabolic flight experiment on sweet potato and barley leaves (Kitaya et al. Citation2001). As a result, leaf transpiration is decreased, causing an increase in leaf temperature. Kitaya et al. reported a mean increase of 1°C on leaf temperature during a 20-second microgravity phase of a parabola (Kitaya et al. Citation2003a). A later parabolic flight experiment confirmed that the lack of natural convection in microgravity slowed down water vapour transfer, which decreased transpiration rate of plant leaves by 46% when gravity levels decreased from 1 g to 10−2 g, and increased the rate by 32% when gravity levels increased from 1 g to 2 g (Hirai and Kitaya Citation2009).
The lack of convection also leads to accumulation of gases and volatile organic compounds, resulting sometimes in very high ethylene concentrations, which is detrimental to plant growth (Musgrave Citation2002; Monje et al. Citation2003). High ethylene concentrations (i.e. 100–1000 parts per billion) can inhibit root and hypocotyl growth because ethylene is phytotoxic and disrupts or induces a variety of metabolic pathways (Levinskikh et al. Citation2000). Therefore, adequate ventilation is mandatory for regular plant development in microgravity and to ensure vital functions such as germination and plant reproduction (Monje et al. Citation2003; Monje, Stutte and Chapman Citation2005). Studying the gaseous environment surrounding plants and including it in the modelling effort of plant growth is crucial for optimal food production in space.
Morphological changes
Many flight experiments demonstrated that plants develop normally in microgravity (Ferl et al. Citation2002), i.e. the overall plant architecture is not altered by the lack of gravity, as long as adequate ventilation is provided. However, the pattern of root growth is more random than what is observed in ground controls, especially lateral roots tend to develop much more in microgravity instead of having a large primary root (Ferl et al. Citation2002) and V. Legué (personal communication, April 2015).
Plants tropisms have been extensively studied in orbit. For example, it was shown that Arabidopsis thaliana seedlings had a greater phototropic response to blue light in microgravity than in Earth gravity (Millar et al. Citation2010). Studies of plant roots in weightlessness have revealed much about the metabolic pathways of gravitropism and how plants grow according to the direction of the gravity vector, as well as about the mechanism of graviresistance, which is sensitive to the magnitude of this gravity vector (Wolverton and Kiss Citation2009; Herranz and Medina Citation2014). It was also shown that roots keep their skewing and waving pattern in microgravity (Paul, Amalfitano and Ferl Citation2012) although this was thought to be an effect of gravity. This experiment also showed that with a directional light, roots of plants grown in orbit are strongly negatively phototropic and grow in the opposite direction to the shoot, as is observed in 1 g on Earth (Paul, Amalfitano and Ferl Citation2012).
Understanding the different tropism mechanisms in plants and how they influence plant growth and development will enable us to use plants more efficiently for food production in future long-duration missions.
Existing solutions and remaining challenges for growing plants in space
The first seeds in space flew aboard the soviet Sputnik 4 in 1960 and the first plant growth system flew aboard the US Biosatellite II in 1967 with four pepper plants (Porterfield et al. Citation2003). Plant growth hardware which has flown in space until now had a weight in the order of a few kilograms and a power consumption in the order of the tens of watts (Porterfield et al. Citation2003). Plant-related experiments were intended to demonstrate the capability of plants to grow in a spacecraft environment and to study plant growth mechanisms, but they were not aimed at large-scale food production.
Watering and nutrient-delivery systems
An efficient and viable watering and nutrient-delivery system (NDS) in microgravity needs to provide water, nutrients and adequate aeration, as well as support plant growth from seed to harvest, while respecting operational and safety constraints of a spacecraft, especially triple nutrient solution containment (Stutte et al. Citation2011). Therefore substrate-based NDS have been used in microgravity instead of aeroponic or hydroponic systems (Monje et al. Citation2003). Moreover it was shown that moisture distribution in microgravity substrate-based NDS is similar to that of a hydroponic system in 1 g (Monje et al. Citation2003). A small-grained soil enables good water distribution in the root zone but prevents good soil aeration and a large grain soil enables proper aeration but voids between grains tend to be filled by air, not only water, which leads to poor root hydration (Casado Citation2006). A trade-off on particle size is therefore necessary and grain sizes used in microgravity-based NDS have ranged from < 0.5 mm to > 5 mm, the most commonly used being zeoponic particles with size 0.5–1 mm and arcillite with sizes 1–2 mm (Monje et al. Citation2003).
In addition, a nutrient-rich solid substrate or slow-release fertilizer is preferred to a nutrient solution, because it limits crew time spent on mixing fresh nutrient solution or making sure the solution is recirculated (Monje et al. Citation2003).
The lack of buoyancy-driven convection in microgravity prevents an efficient nutrient delivery and aeration of the roots. Due to capillary forces, water in microgravity tends to accumulate around water supply tubes, preventing it from reaching the roots uniformally and making nutrient delivery difficult (Dreschel and Sager Citation1989; Casado Citation2006). Early plant growth hardware, such as the soviet Oasis series, suffered from poor water distribution and lack of aeration in the roots (Porterfield et al. Citation2003). Later, systems such as the American Astroculture or the Russian Svet, used porous tubes within nutrient-charged zeolite granules to deliver water and nutrient to the plants by capillarity (Porterfield, Wright and Bausch Citation1984; Morrow et al. Citation1994). Nowadays most microgravity NDS use porous tubes or drip irrigation for water delivery (Monje et al. Citation2003). Control and monitoring of moisture distribution in the growth media have also enabled better growth of plants in microgravity (Monje et al. Citation2003). The most recent plant cultivation system onboard the ISS, Veggie, is composed of pouches with rooting media and controlled-release fertilizer; water is delivered to the pouches using a syringe (Stutte et al. Citation2011), see Table .
Table 1. Significant examples of microgravity plant growth hardware – nutrients and water-delivery system and lighting system characteristics (Ivanova et al. Citation1997; Porterfield, et al. Citation2003; Casado Citation2006; Stutte, et al. Citation2011).
Watering systems for planetary and moon surfaces can take advantage of the reduced gravity and be similar to terrestrial NDS and hence hydroponic (especially nutrient-film technique) and aeroponic systems can be used. For these systems to be efficient in plant growth, the use of real-time ion-specific sensors will be necessary, to monitor and control individual ions in the nutrient solution (Bamsey et al. Citation2012). Indeed plant nutrient uptake is not constant and depends on the crop species and cultivar, the growth phase, and the environment. Such systems have not yet been tested in space but are being developed (Bamsey, Berinstain and Dixon Citation2012).
Lighting
In the early years of plant growth in Earth orbit, lighting was provided by fluorescent lamps (Table ). For example, on the Russian plant growth hardware Oasis 1, which flew on Salyut 1, fluorescent lamps supplied 50 to 68 μmol/m²/s to Brassica capitata, Linum usitatissimum, and Allium porrum plants (Porterfield et al. Citation2003). Today, the American Veggie system uses Light-Emitting Diodes (LED) to illuminate plants on the ISS (Stutte et al. Citation2011). In Citation2006, Massa et al. performed an extensive review of plant growth lighting in space and concluded that LED lights were the future of plant growth lighting in space (Massa et al Citation2006). Indeed LEDs are small, light, have a long lifetime, and a cool emitting surface, and are solid-state light sources, which makes them ideal candidates for plant lighting in space (Bourget Citation2008). Plant productivity is directly related to light intensity, and in order to reduce the planted surface, significant amounts of energy are required (Wheeler et al. Citation2003; Casado Citation2006). A recent study showed that using LED lights instead of traditional High Pressure Sodium or fluorescent lamps can reduce power use per unit of growing area up to one order of magnitude and that the coupled use of targeted close-canopy lighting and spectral optimization with LED lights could significantly reduce energy costs for plant lighting in space and on Earth (Poulet et al. Citation2014a). Furthermore past studies have shown that red and blue wavelengths are best absorbed by plants (McCree Citation1971/1972) and so could mitigate the need for high-intensity lighting. Blue light is less efficient than red light from a photosynthetic point of view but it is very important for plant photomorphogenesis, like stem elongation and leaf expansion (Hoenecke, Bula and Tibbitts Citation1992; Dougher and Bugbee Citation2001).
Saving energy could also be achieved by taking advantage of sunlight on the surface of the planets and the moons. Sun collection systems composed of parabolic mirrors collecting sunlight and optic fibres transmitting this to a greenhouse module could be used in addition to LED lamps (Nakamura et al. Citation2009; Nakamura, Monje and Bugbee Citation2013). A joint study by the German Space Centre (DLR) and the ESA MELiSSA group showed that it was preferable to use such a hybrid lighting system in a Moon greenhouse module (Poulet et al. Citation2013; Eriksson, Doule and Poulet Citation2014).
Ventilation and controlled environment
On the ISS, ethylene concentrations are maintained below 50 ppb, but even such low concentrations have been shown to impact crop yields significantly (Monje et al. Citation2003). The European Modular Cultivation System is equipped with a precise atmospheric control system that removes ethylene from the experiment and controls temperature, humidity and air composition (ESA Citation2013), enabling small-scale and short-term fundamental plant physiology studies. Bigger-scale systems like Lada or Veggie, which are prototypes of food production systems, use cabin-air, which does not enable the collection of accurate data on gas exchange. There is a remaining gap in the know-how and knowledge of large-scale food production systems in microgravity and reduced gravity environments. This is because only small-scale plant growth systems have been taken into orbit.
Confined space
Available room in a spacecraft or in a planetary module is much reduced. Therefore it is critical to select plants and cultivars with high yields but reduced size (De Micco et al. Citation2012). Utah State University has been doing research on dwarf plants for many decades and has developed and tested seeds of dwarf wheat, dwarf cherry tomato, dwarf rice, dwarf pepper, dwarf soybean and dwarf pea, which have successfully grown on orbit and in analogue planetary habitats (Salisbury Citation1997; Poulet et al. Citation2014b). In addition, research is currently on-going on controlled-environment cultivation of dwarf plum trees at Kennedy Space Center (Graham et al. Citation2015). A solution for future long-duration space missions could be to genetically engineer plants and create cultivars that are specifically adapted for confined space and spaceflight environment.
Parameters that need to be observed
A key point for managing growth of plants in space for food production is being able to predict plant yield and accurately control plant growth and development in a spacecraft environment. This can only be achieved with a thorough understanding of the intricate combination of the biochemical, physical and morphological phenomena that govern plant growth in weightlessness. Such knowledge can be ensured with the development of specific experiments and mechanistic and knowledge models of plant growth in reduced gravity environments, which could be validated with the observation and local measurement of a certain set of parameters (see Table ). Hence such an approach would lead to accurate mass and energy balances of the plant (Hezard Citation2012). Water transfer within a plant has proven to be critical for understanding plant growth mechanisms and this needs an accurate evaluation of water absorption at the root level and water transpiration at the leaf surface. The estimation of water content within plant tissue can be achieved by measuring wet and dry masses in destructive sampling. Carbon cycle and carbon balance within a plant are also critical, as this is directly linked to biomass production (Wheeler Citation2003). Carbon fixation through photosynthesis and carbon release through respiration, therefore need to be accurately measured. Carbon dioxide and water vapour release at the leaf surface are linked to the aerodynamic profile of the surrounding air and of the mass boundary layer around the leaf (Kitaya et al. Citation2003b). In order to predict gas exchanges at the leaf surface, it is critical to accurately characterize and describe the aerodynamic profile of the air surrounding the leaf.
Table 2. Parameters that need to be measured to mechanistically model plant growth in reduced gravity environments.
Conclusion
So far, plant experiments in space have focused on the feasibility of plant growth and/or on the study of specific fundamental mechanisms of plant growth and development, and only a few experiments have accurately locally measured inputs and outputs within a plant.
Effects of spaceflight on plant growth are not yet fully understood but it seems that it causes no major obstacle to plant growth in space, as long as adequate ventilation, lighting, and temperature and humidity control are provided. However, large-scale tests for food production in reduced gravity are still lacking. Research and technological tests on optimal nutrient-delivery systems for microgravity or partial gravity are still on-going; lighting for plant growth in space has progressed tremendously since the 1980s but the energetic burden generated by electrical lighting remains a limit for large-scale plant production; much work remains to be done on plant gas-exchange in reduced gravity environments, especially when it comes to larger growth chambers; the limited amount of room in a spacecraft drives the choice of plant species towards small crops, with a high harvest index, and cultivar selection for food production in space currently is on-going; finally growth mechanisms under space factors need to be fully understood to accurately predict the behaviour of biological processes and have a reliable LSS based on higher plants. Therefore developing mechanistic models of plant growth subjected to space environment (e.g. reduced gravity, high radiations, changing magnetic field, and low pressure) is crucial, as it helps us to understand underlying mechanisms and identify knowledge gaps in plant growth and development.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Lucie Poulet worked for 2 years at the German Aerospace Center Institut of Space Systems (prior to starting her PhD at University Blaise Pascal) where she acquired experience in managing European Space Agency (ESA) projects, as well as participating in and leading concurrent engineering studies. Her experience in the 4-month simulated Mars mission HI-SEAS mission and at Purdue University during her Master’s degree in aerospace engineering has led her to acquire competence in designing plant-related experiments and identifying flaws in hardware for plant growth in controlled environments. Focusing on developing physical models of higher plant growth in reduced gravity environment led her to review how plants grow in space and how microgravity influences their growth and development, as well as what are the remaining challenges on this topic. Contribution: Lucie Poulet’s work at the University Blaise Pascal is focused on developing physical models of higher plant growth in reduced gravity environment. This work led her to review how plants grow in space and how microgravity influences their growth and development, as well as what are the remaining challenges on this topic.
J.-P. Fontaine has focused on the modelling of complex multi-physics phenomena: non-linear, coupled processes, multi-scale, hydrodynamic convective instabilities, rotating flows, interfacial phenomena (moving boundaries, thermocapillary flows), phase changes (evaporation/condensation, solidification), reduced gravity environments, experiments/modelling interface and industrial/engineering-related problems. He has worked on the implementation of numerical methods (finite elements, finite volumes, finite differences) related to transport phenomena: fluid, heat, species, ions, light. He also has experience in the characterization of thermophysical properties of materials. He is currently concentrating on a precise characterization of the heterogeneous transfer occurring at interfaces during condensation phases and at the modelling of the influence of gravity on higher plant growth, particularly in reduced gravity. Contribution: as one of L. Poulet’s PhD supervisor, offering necessary directions and insights to assist in her research, as well as revisions on this paper.
C.-G. Dussap has been much involved in the analysis of the relationships that exist between the physiological responses of microorganisms and the bioreactor environment. This includes insightful investigation in metabolic engineering (metabolic fluxes distribution, control and regulation of metabolism) and thorough analysis of bioreactor performances regarding the mass, heat, light-energy transfer and mixing properties of reactors. He has strong experience in mathematical modelling of biological kinetics, thermodynamic equilibrium properties of aqueous solutions and of reactor characteristics. He has a track record of experience in the design and the mathematical modelling of the MELiSSA (Micro-Ecological Life Support System Alternative) ecosystem developed by the ESA for long-duration space missions. He has been involved as UBP representative in the ESA Memorandum of Understanding concerning MELiSSA system since its start in 1993. His main domain of expertise covers all the process engineering aspects of MELiSSA, especially the bioreactor design, modelling, scale-up and control. This includes for his research team the management of the experimental assays and of the studies, which are performed for ESA in various European centres Ghent, Barcelona, Clermont-Ferrand, Guelph...etc.). He is the author of more than 150 international publications. Contribution: as one of L. Poulet’s PhD supervisor, offering necessary directions and insights to assist in her research, as well as revisions on this paper.
Acknowledgements
The authors would like to thank the Centre National d’Etudes Spatiales (CNES) and Centre National de Recherche Scientifique (CNRS) who supported this work.
References
- Arena, C., V. De Micco, E. Macaeva, and R. Quintens. 2014. “Space radiation effects on plant and mammalian cells.” Acta Astronautica 104: 419–431.
- Bagdigian, R. M., and D. Cloud. 2005. “Status of the International Space Station Regenerative ECLSS Water Recovery and Oxygen Generation Systems.” Society of Automotive Engineers, Inc. 2005-01-2779 (2005).
- Bamsey, M., A. Berinstain, and M. Dixon. 2012. “Development of a potassium-selective optode for hydroponic nutrient solution monitoring.” Analytica Chimica Acta 737: 72–82.
- Bamsey, M., T. Graham, C. Thompson, A. Berinstain, A. Scott, and M. Dixxon. 2012. “Ion-Specific Nutrient Management in Closed Systems: The Necessity for Ion-Selective Sensors in Terrestrial and Space-Based Agriculture and Water Management Systems.” Sensors (Basel) 12 (10): 13349–13392.
- Bourget, C. M. 2008. “An introduction to light emitting diodes.” HortScience 43 (7): 1944–1946.
- Brown, C. S., W. C. Piastuch, and W. M. Knott. 1994. “Soybean cotyledon starch metabolism is sensitive to altered gravity conditions.” Advances in Space Research 14 (8): 107–110.
- Burkey, R. C., S. T. Green, S. P. Siebenaler, and J. C. Buckingham. 2010. “From CO2 to H2O.” Technology Today, Summer 2010: 6–9.
- Carter, D. L. 2010. Status of the Regenerative ECLSS Water Recovery System. Marshall Space Flight Center: NASA.
- Casado, J. 2006. “Cultivating the Future – Growing food in space.” Spaceflight, May 2006: 180–189.
- Cogne, G., J.-B. Gros, and C.-G. Dussap. 2003. “Identification of a metabolic network structure representative of Arthrospira (Spirulina) platensis metabolism.” Biotechnology and Bioengineering 84 (6): 667–676.
- Cornet, J.-F., C. G. Dussap, and J.-B. Gros. 1998. “Kinetics and energetics of photosynthetic micro-organisms in photobioreactors: application to Spirulina growth.” Advances in Biochemical Engineering/Biotechnolog 59: 153–224.
- De Micco, V., G. Aronne, and S. De Pascale. 2006. “Effect of simulated microgravity on seedling development and vascular differentiation of soy.” Acta Astronautica 58: 139–148.
- De Micco, V., and G. Aronne. 2008. “Biometric anatomy of seedlings developed onboard of Foton-M2 in an automatic system supporting growth.” Acta Astronautica 62: 505–513.
- De Micco, V., C. Arena, D. Pignalosa, and M. Durante. 2011. “Effects of sparsely and densely ionizing radiation on plants.” Radiation and Environmental Biophysics 50: 1–19.
- De Micco, V., R. Buonomo, R. Paradiso, S. De Pascale, and S. Aronne. 2012. “Soybean cultivar selection for Bioregenerative Life Support Systems (BLSS) – Theoretical selection.” Advances in Space Research 49 (10): 1415–1421.
- De Micco, V., S. De Pascale, R. Paradiso, and G. Aronne. 2014. “Microgravity effects on different stages of higher plant life cycle and completion of the seed-to-seed cycle.” Plant Biology 16 (1): 31–38.
- Dougher, T. A. O., and B. Bugbee. 2001. “Differences in the response of wheat, soybean and lettuce to reduced blue radiation.” Photochemistry and Photobiology 73 (2): 199–207.
- Dreschel, T. W., and J. C. Sager. 1989. “Control of water and nutrients using a porous tube: a method for growing plants in space.” Horticultural Science 24 (6): 944–947.
- Eckart, P. 1994. Life Support and Biospheric. Munich: Herbert Utz publishers.
- Eriksson, K., O. Doule, and L. Poulet. 2014. “Architectural Concepts For A Lunar Greenhouse Within The Melissaframework.” 65th International Astronautical Congress. Toronto, Canada, 2014.
- ESA. 2013. “European Modular Cultivation System (EMCS).” ESA-HSO-COU-013, 2013.
- Ferl, R., R. Wheeler, H. G. Levine, and A. L. Paul. 2002. “Plants in space.” Current Opinions in Plant Biology 5 (3): 528–563.
- Giardi, M. T., G. Rea, M. D. Lambreva, A. Antonacci, S. Pastorelli, I. Bertalan, U. Johanningmeier, and A. K. Mattoo. 2013. “Mutations of Photosystem II D1 protein that empower efficient phenotypes of Chlamydomonas reinhardtii under extreme environment in space.” PLOS One 8, no. 5 ( 2013).
- Gitelson, I. I., I. A. Terskov, B. G. Kovrov, G. M. Lisovsky, Y. N. Okladnikov, F. Y. Sid'ko, I. N. Trubachev, et al. 1989. “Long-term experiments on man's stay in biological life-support system.” Advances in Space Research 9 (8): 6–71.
- Gitelson, I. I., and G. M. Lisovsky. 2008. “Creation of Closed Ecological Life Support Systems: Results, Critical Problems and Potentials.” Journal of Siberian Federal University Biology 1: 19–39.
- Gòdia, F., J. Albiol, J. L. Montesinos, J. Pérez, N. Creus, F. Cabello, X. Mengual, A. Montras, and C. Lasseur. 2002. “MELiSSA: a loop of interconnected bioreactors to develop life support in Space.” Journal of Biotechnology 99 (3): 319–330.
- Graham, T., R. Scorza, R. M. Wheeler, C. Dardick, B. Smith, A. Dixit, D. Raines, et al. 2015. “Over expression of FT1 in plum (Prunus domestica) results in phenotypes compatible with spaceflight: A new candidate crop for bioregenerative life-support systems.” Gravitational and Space Research 3 (1): 39–50.
- Herranz, R., and F. J. Medina. 2014. “Cell proliferation and plant development under novel altered gravity environments.” Plant Biology 16 (1): 23–30.
- Hezard, P. 2012. Higher Plant Growth Modelling for Life Support Systems: Global Model Design and Simulation of Mass and Energy Transfers at the Plant Level. PhD Thesis, Clermont Ferrand: Doctoral school of Life Sciences, Health, Agronomy, Environment – Université Blaise Pascal, Université d'Auvergne, 2012.
- Hezard, P., L. S. Sasidharan, C. Creuly, and C.-G. Dussap. 2010. “Higher plant modeling for bioregenerative life support applications: General structure of modeling.” Proceedings of the 40th International Conference on Environmental Systems. Barcelona, Spain, 2010.
- Hezard, P., S. Sasidharan, C.-G. Dussap, C. Creuly, and L. Poughon. 2012. “Predicting and controlling higher plant growth following mass and energy balances approach: a review and new model design.” Functional Plant Biology, 2012
- Hirai, H., and Y. Kitaya. 2009. “Effects of Gravity on Transpiration of Plant Leaves.” Interdisciplinary Transport Phenomena: Annals of the New York Academy of Science 1161: 166–172.
- Hoenecke, M. E., R. J. Bula, and T. W. Tibbitts. 1992. “Importance of blue photon levels for lettuce seedlings grown under red light-emitting diodes.” HortScience 27 (5): 427–430.
- Ivanova, T., S. Sapunova, P. Kostov, and I. Dandolov. 1997. First Successful Space Seed-to-Seed Plant Growth Experiment in the SVET-2 Space Greenhouse in 1997. Sofia, Bulgaria: Space Research Institute, Bulgarian Academy of Sciences.
- Kiss, J. Z., G. Aanes, M. Schiefloe, L. H. F. Coelho, K. D. L. Millar, and R. E. Edelmann. 2014. “Changes in operational procedures to improve spaceflight experiments in plant biology in the European Modular Cultivation System.” Advances In Space Research 53 (5): 818–827.
- Kitaya, Y., M. Kawai, J. Tsuruyama, H. Takahashi, A. Tani, E. Goto, T. Saito, and M. Kiyota. 2001. “The effect of gravity on surface temperature and net photosynthetic rate of plant leaves.” Advances in Space Research 28 (4): 659–664.
- Kitaya, Y., M. Kawai, J. Tsuruyama, H. Takahashi, A. Tani, E. Goto, T. Saito, and M. Kiyota. 2003a. “The effect of gravity on surface temperatures of plant leaves.” Plant, Cell And Environment 26: 497–503.
- Kitaya, Y., J. Tsuruyama, T. Shibuya, M. Yoshida, and M. Kiyota. 2003b. “Effects of air current speed on gas exchange in plant leaves and plant canopies.” Advances in Space Research 31 (1): 177–182.
- Kittang Jost, A.-I., T. Hoson, and T.-H. Iversen. 2015. “The Utilization of Plant Facilities on the ISS the composition, growth, and development of plant cell walls under microgravity conditions.” Plants 5: 44–62.
- Known, T., J. A. Sparks, J. N. Nakashima, S. N. Allen, Y. Tang, and E. B. Blancaflor. 2015. “Transcriptional response of Arabidopsis seedlings during spaceflight reveals peroxidase and cell wall remodeling genes associated with root hair development.” American Journal of Botany 102 (1): 21–35.
- Kuang, A., Y. Xiao, and M. E. Musgrave. 1996. “Cytochemical localization of reserves during seed development in arabidopsis thaliana under spaceflight conditions.” Annals Of Botany 78: 343–351.
- Lasseur, C., J. Brunet, H. De Weever, M. Dixon, C.-G. Dussap, F. Godia, N. Leys, M. Mergeay, and D. Van Der Straeten. 2010. “MELiSSA: The European Project Of Closed Life Support System.” Gravitational and Space Biology 23 (2): 3–12.
- Levinskikh, M. A., V. N. Sychev, T. A. Derendyaeva, O. B. Signalova, F. B. Salisbury, W. F. Campbell, G. E. Bingham, D. L. Bubenheim, and G. Jahns. 2000. “Analysis of the spaceflight effects on growth and development of Super Dwarf wheat grown on the Space Station Mir.” Journal of Plant Physiology 156 (4): 522–529.
- Manzano, A. I., I. Matia, F. Gonzalez-Camacho, E. Carnero- Diaz, J. J. W. A. Van Loon, C. Dijkstra, O. Larkin, et al. 2009. “Germination of Arabidopsis seed in Space and in simulated microgravity: alterations in root cell growth and proliferation.” Microgravity Science and Technology 21: 293–297.
- Massa, G. D., J. C. Emmerich, R. C. Morrow, C. M. Bourget, and C. A. Mitchell. 2006. “Plant-growth lighting for space life support: a review.” Gravitational and Space Biology 19 (2): 19–30.
- Mazars, C., C. Briere, S. Grat, C. Pichereaux, M. Rossignol, V. Pereda-Loth, B. Eche, et al. 2014. “Microgravity induces changes in microsome-associated proteins of Arabidopsis seedlings grown on board the International Space Station.” PLoS ONE 9 (3).
- McCree, K. J. 1971–1972. “The action spectrum, absorptance and quantum yield of photosynthesis in crop plants.” Agric. Meteorol. 9 ( 1971-1972): 191–216.
- Medina, J., A. I. Manzano, R. Herranz, C. Dijkstra, O. Larkin, R. Hill, E. Carnero-Díaz, et al. 2010. “Effects of real or simulated microgravity on plant cell growth and proliferation.” 38th COSPAR Scientific Assembly. Bremen, Germany, 2010. 12.
- Medina, F. J., R. Herranz, C. Arena, G. Aronne, and V. De Micco. 2015. “Chapter 24: Growing plants under generated extra-terrestrial environments: effects of altered gravity and radiation.” In Generation and Applications of Extra-Terrestrial Environments on Earth, edited by D. A. Beysens and J. J. W. A. Van Loon, 239–254. Aalborg, Denmark: River Publishers.
- Millar, K. D., P. Kumar, M. J. Correll, J. L. Mullen, R. P. Hangarter, R. E. Edelmann, and J. Z. Kiss. 2010. “A novel phototropic response to red light is revealed in microgravity.” New Phytologist 186 (3): 648–656.
- Monje, O., G. Stutte, and D. Chapman. 2005. “Microgravity does not alter plant stand gas exchange of wheat at moderate light levels and saturating CO2 concentration.” Planta 222 (2): 336–345.
- Monje, O., G. W. Stutte, G. D. Goins, D. M. Porterfield, and G. E. Bingham. 2003. “Farming in space: environmental and biophysical concerns.” Advances in Space Research 31 (1): 151–167.
- Morrow, R. C., R. J. Bula, T. W. Tibbitts, and W. R. Dinauer. 1994. “The astroculture tm flight experiment series, validating technologies for growing plants in space.” Advances in Space Research 14 (11): 29–37.
- Musgrave, M. E. 2002. “Seeds in Space.” Seed Science Research 12 (1): 1–16.
- Musgrave, M. E., A. Kuang, and S. W. Matthews. 1997. “Plant reproduction during spaceflight: importance of the gaseous environment.” Planta 203: 177–184.
- Musgrave, M. E., A. Kuang, L. K. Tuominen, L. H. Levine, and R. C. Morrow. 2005. “Seed storage reserves and glucosinolates in Brassica rapa L. grown on the International Space Station.” Journal of the American Society for Horticultural Science 130 (6): 848–856.
- Nakamura, T., A. D. Van Pelt, N. C. Yorio, A. E. Drysdale, R. M. Wheeler, and J. C. Sager. 2009. “Transmission and distribution of photosynthetically active radiation (PAR) from solar and electric light sources.” Habitation 12: 103–117.
- Nakamura, T., O. Monje, and B. Bugbee. 2013. “Solar Food Production and Life Support in Sapce Exploration.” AIAA SPACE 2013 Conference and Exposition. San Diego, 2013.
- Ordoñez, L., C. Lasseur, L. Poughon, and G. Waters. 2004. “MELiSSA Higher Plants Compartment modeling using EcoSim Pro.” SAE International 2004-01-2351 ( 2004).
- Paradiso, R., V. De Micco, R. Buonomo, G. Aronne, G. Barbieri, and S. De Pascale. 2014. “Soilless cultivation of soybean for Bioregenerative Life Support Systems (BLSSs): a literature review and the experience of the MELiSSA Project - Food characterization Phase I.” Plant Biology 16: 69–78.
- Paul, A. L., C. L. Amalfitano, and R. J. Ferl. 2012. “Plant growth strategies are remodeled by spaceflight.” Plant Biology 2012: 12–232.
- Paul, A. L., M. P. Popp, W. B. Gurley, C. Guy, K. L. Norwood, and R. J. Ferl. 2005. “Arabidopsis gene expression patterns are altered during spaceflight.” Advances in Space Research 36 (7): 1175–1181.
- Paul, A. L., R. M. Wheeler, H. G. Levine, and R. J. Ferl. 2013. “Fundamental plant biology enabled by the Space Shuttle.” American Journal of Botany 100 (1): 226–234.
- Porterfield, D. M. 2002. “The biophysical limitations in physiological transport and exchange in plants grown in microgravity.” Journal of Plant Growth Regulation 21 (2): 177–190.
- Porterfield, D. M., B. D. Wright, and W. C Bausch. 1984. “A Plant Growth System for Orbital Plant Experiments.” Amer. Soc. Agric. Eng. New Orleans, LA, 1984. Paper # 84-2524.
- Porterfield, D. M., S. W. Matthews, C. J. Daugherty, and M. E. Musgrave. 1997. “Spaceflight exposure effects on transcription, activity, andlocalization of alcohol dehydrogenase in the roots of Arabidopsis thaliana.” Plant Physiology 113 (3): 685–693.
- Porterfield, D. M., G. S. Neichitailo, A. L. Mashinski, and M. E. Musgrave. 2003. “Spaceflight hardware for conducting plant growth experiments in space: the early years 1960–2000.” Advances in Space Research 31 (1): 183–193.
- Poughon, L. 2007. Dynamic Modelling of a Coupled MELiSSA Crew – Compartment C4a with Matlab/Simulink. MELiSSA Technical Note 83.2, ESA/ESTEC CONTRACT, 2007.
- Poughon, L. 1997. Including of a Higher Plants Chamber in the MELiSSA loop Description of a HPC for MELiSSA loop steady state simulations. MELiSSA Technical Note 32.3, ESA/ESTEC CONTRACT, 1997.
- Poulet, L., D. Schubert, C. Zeidler, P. Zabel, V. Maiwald, E. David, and C. Paillé. 2013. “Greenhouse Modules and Regenerative Life-Support Systems for Space.”AIAA SPACE 2013 Conference and Exposition.San Diego, CA, 2013.
- Poulet, L., G. D. Massa, R. C. Morrow, C. M. Bourget, R. M. Wheeler, and C. A. Mitchell. 2014a. “Significant reduction in energy for plant-growth lighting in space using targeted lighting and spectral manipulation.” Life Sciences in Space Research 2: 43–53.
- Poulet, L., G. D. Massa, R. M. Wheeler, T. Gill, C. Steele, R. C. Morrow, T. M. Swarmer, K. Binsted, and J. Hunter. 2014b. “Demonstration Test of Electrical Lighting Systems For Plant Growth in HI-SEAS Analog Mars Habitat .” 65th International Astronautical Congress. Toronto, 2014.
- Rygalov, V., B. G. Kovrov, and G. S. Denisov. 1997. “Interaction of physical-chemical and biological regeneration processes in ecological life support systems.” Advances in Space Research 20 (10): 1995–2000.
- Salisbury, F. B. 1997. “Growing Super-Dwarf wheat in Space Station Mir.” Life Support Biosphere Science 4 (3–4): 155–166.
- Salisbury, F. B., J. I. Gitelson, and G. M. Lisovsky. 1997. “Bios-3: Siberian Experiments in Bioregenerative Life Support.” BioScience 47 (9): 575–585.
- Salmi, M. L., and S. J. Roux. 2008. “Gene expression changes induced by space flight in single-cells of the fern Ceratopteris richardii.” Planta 229 (1).
- Slocum, R. D., J. J. Gaynor, and A. W. Galston. 1984. “Cytological and ultrastructural studies on root tissues.” Annals of Botany 54 (3): 65–76.
- Steinberg, S. L., D. W. Ming, and D. Henninger. 2002. Plant production systems for microgravity: critical issues in water, air, and solute transport through unsaturated porous media. Washington, DC: NASA.
- Stutte, G. W., G. Newsham, R. M. Morrow, and R. M. Wheeler. 2011. “Operational Evaluation of VEGGIE Food Production System in the Habitat Demonstration Unit.” Reston, VA: American Institute of Aeronautics and Astronautics (AIAA), 2011.
- Tuominen, L. K., L. H. Levine, and M. E. Musgrave. 2009. “Plant secondary metabolism in altered gravity.” Methods in Molecular Biology 547: 373–386.
- Vandenbrink, J. P., J. Z. Kiss, R. Herranz, and F. J. Medina. 2014. “Light and gravity signals synergize in modulating plant.” Frontiers in Plant Science 5 (563).
- Vandenbrink, J. P., and J. Z. Kiss. 2016. “Space, the final frontier: a critical review of recent experiments performed in microgravity.” Plant Science 243: 115–119.
- Wheeler, R. M. 2003. “Carbon balance in bioregenerative life support systems: some effects of system closure, waste management, and crop harvest index.” Advances in Space Research 31 (1): 169–175.
- Wheeler, R. M. 2010. “Plants For Human Life Support In Space: From Myers To Mars.” Gravitational and Space Biology 23 (2): 25–35.
- Wheeler, R. M., J. C. Sager, R. P. Prince, W. M. Knott, C. L. Mackowiak, G. W. Stutte, N. C. Yorio, L. M. Ruffe, et al. 2003. Crop Production for Advanced Life Support Systems - Observations From the Kennedy Space Center Breadboard Project. Technical Report, Kennedy Space Center: NASA.
- Wheeler, R. M., C. L. Mackowiak, G. W. Stutte, J. C. Sager, N. C. Yorio, L. M. Ruffe, R. E. Fortson, T. T. W. Dreschel, W. M. Knott, and K. A. Corey. 1996. “NASA's Biomass Production Chamber: a testbed for bioregenerative life support studies.” Advances in Space Research 18 (4–5): 215–224.
- Wolff, S. A., L. H. Coelho, I. Karoliussen, and A. I. Kittang Jost. 2014. “Effects of the extraterrestrial environment on plants: recommendations for future space experiments for the MELiSSA Higher Plant Compartment.” Life 4 (2): 189–204.
- Wolverton, C., and J. Z. Kiss. 2009. “An Update on Plant Space Biology.” Gravitational and Space Biology 22 (2): 13–20.
- Zhang, Y., L. Wang, J. Xie, and H. Q. Zheng. 2015. “Differential protein expression profiling of Arabidopsis thaliana callus under microgravity on board the Chinese SZ-8 spacecraft.” Planta 241: 475–488.
