Abstract
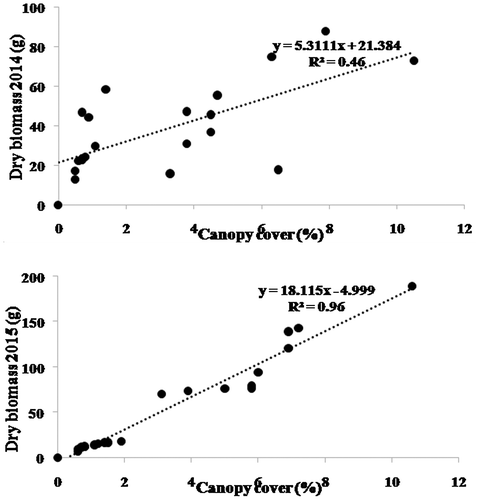
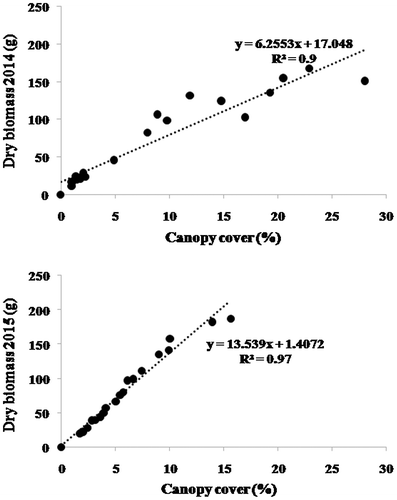
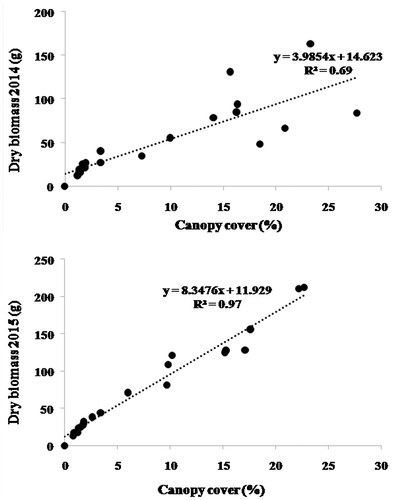
Plant biomass is usually assumed to be positively correlated with canopy cover. Measurements of canopy cover can now be easily and precisely made through image processing of high-resolution digital photography. This study aims to determine rapid, accurate and non-destructive ecological measurements of the aboveground biomass of selected plant species of the dry rangelands of southern Tunisia. The digital vegetation charting technique was used to estimate the canopy cover of three plant species: Artemisia herba-alba Asso., Rhanterium suaveolens Desf. and Stipa tenacissima L. Biomass of the selected species was also determined to develop regression models and correlation equations with the relative canopy cover. The main result was a strong correlation between canopy cover and dry biomass: coefficients of determination of 0.46 and 0.96 for A. herba-alba during the springs of 2014 and 2015, respectively; and correspondingly 0.9 and 0.97 for R. suaveolens and 0.69 and 0.97 for S. tenacissima. This digital charting technique offers a non-destructive, accurate and rapid means for monitoring and assessment of rangeland productivity dynamics as well as an efficient tool for developing range management strategies in dry areas.
Introduction
Grazing influences the plant community structure and productivity in rangelands (Wu et al. Citation2009). Changes in vegetation and soils under heavy continuous grazing have been widely documented around the world (Louhaichi, Ghassali, et al. Citation2012; Mekuria and Aynekulu Citation2013; Angassa Citation2014), and the impact depends mainly on climate conditions and vegetation types (Palacio, Bisigato, and Bouza Citation2014). Vegetation height, coverage and productivity decrease significantly with increasing grazing intensity (Wu et al. Citation2009).
Restoration techniques enhance the recovery and improvement of all rangeland conditions (Mureithi et al. Citation2014). Fencing (preventing grazing by means of fencing herbivores out) is one of the best management practices to restore rangeland plant communities and improve their aboveground productivity (Wu et al. Citation2011; Scanlan et al. Citation2014). Rangeland managers are interested in evaluating the impact of managerial actions on vegetation and it is important for them to be able to document the ecosystem conditions and trends in relation to such actions (Chaichi, Saravi, and Malekian Citation2005; Louhaichi et al. Citation2010). The use of digital vegetation-cover maps covering large areas can provide valuable information for land managers about the spatial and temporal variability of rangeland sites at landscape level (Wiles Citation2011; Bauer and Strauss Citation2014). Traditionally, rangeland scientists have employed a laborious technique of quadrat sampling for vegetation measurements in the field (Barbour, Burk, and Pitts Citation1987; Cox Citation1990; Frank and McNaughton Citation1990; Hill et al. Citation2005; Louhaichi, Salkini, and Petersen Citation2009; Louhaichi et al. Citation2010). These conventional techniques are difficult, time consuming, expensive and have resulted in detailed records for very few locations worldwide (Radloff and Mucina Citation2007; Louhaichi et al. Citation2010). Furthermore, the accuracy and efficiency of using the digital vegetation charting technique for estimating cover are far better than visual estimates (Olmstead et al. Citation2004; Preuss et al. Citation2012).
Cooper (Citation1924) reported the first use of vertical photography for plant cover analysis using a wooden camera-stand to acquire photographs of permanent plots. Since 1924, a succession of camera-stand designs has been used in rangeland vegetation studies (Booth et al. Citation2004). Photographic equipment and electronics have evolved rapidly but the photographic monitoring methods for rangelands have changed little over time because each picture is a compromise between resolution and area covered (Booth et al. Citation2005). Image-analysis software and applications have been developed for determining rangeland ground cover from colour digital images (Louhaichi et al., “Native Forbs”, Citation2012; Louhaichi et al. Citation2013; Luo et al. Citation2015). There are several image processing software programs able to calculate ground cover. One of these programs, VegMeasure, was developed at Oregon State University (USA) in partnership with the International Center for Agricultural Research in the Dry Areas (ICARDA). This software is used to measure plant cover on rangelands and to draw the vegetation map for a sample quadrat on the ground, using pictures taken vertically downwards (Louhaichi, Borman, and Johnson Citation2001; Louhaichi et al. Citation2010; Louhaichi et al., “Developing a coherent monitoring system”, Citation2012; Johnson et al. Citation2015; VegMeasure Citation2015). The program quantifies areas of each defined class such as bare ground and vegetation using built-in algorithms. The software allows patch processing of multiple images either through binary or supervised classifications of colour images.
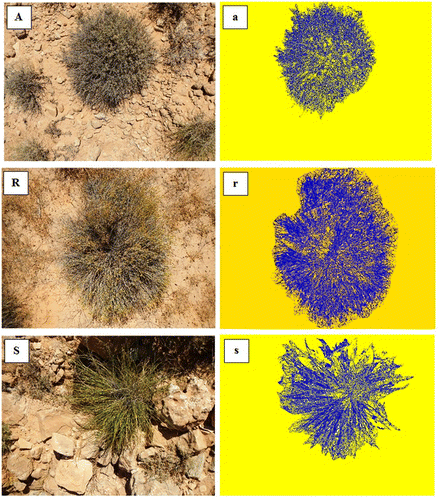
Plant biomass is believed to be positively correlated with the relative canopy cover (Lefsky et al. Citation2002; Booth et al. Citation2006; Laliberté et al. Citation2007). Biomass is usually determined by one of the three following techniques: clipping, direct estimation and dimensions analyses in the field. However, plant material is rare and with higher environmental value in dry areas. For this reason, much effort is made to protect, conserve and decrease the degradation of these natural resources. Research on plant material is also focused on non-destructive techniques. In this study, a photograph monitoring technique was applied to speed up the collection of information concerning plant biomass using high-resolution digital photography combined with image processing. Three plant species (Artemisia herba-alba Asso., Rhanterium suaveolens Desf. and Stipa tenacissima L.) were selected for a number of reasons: (1) they represent the main key steppe species in arid areas of North Africa, (2) they are endangered and have become rare due to their palatability, traditional uses and location (their biotope is used for agricultural practices), and (3) they grow in different habitats within the same site (mountain, sloping and flat areas).
Material and methods
Study site
This study was carried out in a private rangeland located near Beni Khedeche (El Bhayra), in the Matmata Mountain Chain of the southern dry area of Tunisia (33°18'27.2" N, 10°13'15.79" E). Plant communities of the whole region are dominated by S. tenacissima, A. herba-alba and R. suaveolens. Covering an area of 20 ha, the study site is characterized by diverse geomorphological landscapes: mountain, sloping areas and plains. It has been excluded from livestock grazing since March 2012.
Experimental design
The biomass and canopy cover for S. tenacissima, A. herba-alba and R. suaveolens were measured during the springs of 2014 and 2015. Twenty tufts of each species were selected for measurements: 10 of medium size in the fenced area, and 10 of small size in the area open to grazing. The aboveground biomass was obtained by clipping half of the aerial parts of the selected plants. The plant material was weighed, dried at 105 °C for 24 h and then re-weighed to obtain the dry biomass (BM). The tufts concerned were photographed with a high-resolution digital camera before and after clipping. The same camera height above ground (1.25 m) was kept during the protocol (i.e. the photographed area was the same). The camera has to be pointed vertically downward (a bubble level ensured this) and the directional orientation was known (a compass permitted the top of the camera to be pointed in a certain direction for each image). It was preferable to point either to the south or to the north in order to eliminate the equipment shadow from photographs. The photographs taken before clipping were used in this work, and were analysed with VegMeasure software to determine the canopy cover (CC) of the targeted species (Figure ). The CC was expressed as a percentage of the plant cover in the photographed area.
Results
Artemisia herba-alba
There was a significant correlation between CC and BM for A. herba-alba (Figure ). The linear regression between BM and CC was significant: coefficient of determination (R²) was 0.46 and 0.96 in spring of 2014 and 2015, respectively. The BM values were in the range of 12.8–87.8 g (average 39.4 g) in the springs of 2014. In 2015, the respective values were 6.6–189.5 g (average 59.78 g). The CC values were 0.5–10.5% (3.2% on average) in 2014 and 0.6–10.6% (3.6% on average) in 2015.
Rhanterium suaveolens
There was a significant correlation between BM and CC for R. suaveolens (Figure ). R² of the linear regression model between BM and CC was about 0.90 and 0.97 in the springs of 2014 and 2015, respectively. The average of BM was 74.3 and 82.7 g in the springs of 2014 and 2015, respectively; the corresponding average CCs were 9% and 6%.
Stipa tenacissima
Linear regressions were obtained between BM and CC for S. tenacissima during the springs of 2014 (R² = 0.69) and 2015 (R² = 0.97) (Figure ). For the 20 measured tufts, BM was in the range of 12 g (minimum) and 163 g (maximum) (with average of 53 g); the corresponding CC values were 1.2 and 27% (with average of 9.5%).
Discussion
Two of the most important indicators for rangeland health are CC and productivity (Pelland et al. Citation2000). In this study, BM was positively correlated with CC for the three studied species, confirming the results of Laliberté et al. (Citation2007) and Inoue et al. (Citation2015). The R² of the regression relationships between BM and CC were 0.46, 0.90 and 0.69 for A. herba-alba, R. suaveolens and S. tenacissima in spring 2014, respectively. It is advantageous to have a measure of canopy vegetation cover that correlates well with dry biomass. Ideally, if a method has a high positive correlation with aboveground biomass, then destructive sampling is unnecessary (Marsett et al. Citation2006). The present results suggest such a relationship and could be used to estimate cover without destructive sampling. According to Gilbert and Butt (Citation2009), digital imagery measurements of vegetation cover showed no significant difference compared with a traditional point–quadrat method (widely accepted for estimating vegetation cover). Hence, this digital technique can be implemented on a large scale because it is easy to use, the camera stand is lightweight, small and practical, and the photographs can be taken rapidly (see the description above).
Since the beginning of range management as a discipline, the evaluation and monitoring of expansive landscapes have relied more on judgement and experience than science. The challenge is to develop cost-effective methods for detecting important vegetation changes with acceptable error rates. Improvement in computers, image-analysis software, cameras and camera platforms are rapidly expanding the vegetation-monitoring capability. In recent years, digital photography has become popular, easy to use and an affordable tool for the scientific community to document and present scientifically sound findings. In particular, high-resolution digital images are useful for several types of data gathering and have proven to be a quick and accurate means for vegetation classification (Luscier et al. Citation2006; Louhaichi et al. Citation2010; Barrachina, Cristóbal, and Tulla Citation2015). It is important to note that developing an experimental protocol for measuring vegetation requires careful assessment of management goals in relation to the benefits received from sampling efforts (Louhaichi et al. Citation2010; Larson et al. Citation2013). Image-based collection methods can be faster once they are fully developed. They have the added advantage that photographs can be acquired quickly at different times to assess vegetation dynamics for monitoring purposes. In addition, photographs are easily archived and conserve a maximal data for future change analyses (Laliberté et al. Citation2007).
Nevertheless, there are limitations with use of the proposed technique. Since the images are taken vertically, BM of species with long upright form may be underestimated, but overestimated for species with horizontal growth. To overcome such a handicap, additional variables such as height should be considered. Since this technique targets only individual plant species, an estimate of the number of plants present in the site is needed. For improved sites, this can be easily deduced based on the density per hectare. Once the number of shrub species is known, this can be simply multiplied by the estimated biomass per plant to determine total shrub biomass for the whole site.
Conclusions
Accurate measurement of canopy vegetation cover is a key parameter to calculate rangeland productivity. In this study, a digital non-destructive method to estimate the plant BM of selected rangeland plant species was tested. The correlations between BM and CC were developed using digital photography combined with image processing and conventional clipping for calibration purposes. The results showed a high level of accuracy and ease of use for this technique. This study demonstrated the benefits and shortcomings of using this, a non-destructive method, for quantification of the plant BM in dry pastoral ecosystems. In future research, this technique can be used to evaluate the impact of biotic and abiotic stresses such as climatic changes and human impacts on vegetation. It will also be beneficial to fine-tune this technique for other plant species present in Saharan and other arid areas.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Mohamed Tarhouni PhD in biological sciences and plant ecology. He is a permanent researcher at the Institut des Régions Arides of Medenine (Tunisia). He has supervised many MSc theses and teaches ecology, botany and phytosociology in Tunisian universities. Contribution: Contributions to the idea and experimental design of this study; setup of the design, the data collection and analysis; writing and interpretation of results and discussion and notable contribution to final revision.
Farah Ben Salem PhD in agronomy and rangeland ecology. He is a full time researcher at the Institut des Régions Arides of Medenine. Contribution: Contributions to the idea and experimental design of this study; setup of the design, the data collection and analysis,
Abderrazak Tlili PhD student in biology and plant ecology at the Institut des Régions Arides of Medenine. He has a forestry undergraduate degree, a MSc in life and earth sciences, biology and environment. Contribution: Contributions to the idea and experimental design of this study; setup of the design, the data collection and analysis were performed mainly by the three first authors; writing and interpretation of results and discussion
Azaiez Ouled Belgacem Professor in rangeland ecology and management. He is the Regional Coordinator of the Arabian Peninsula Regional Program of the International Center for Agricultural Research in the Dry Areas (ICARDA). Contribution: Contributions to the idea and experimental design of this study.
Mohamed Neffati Professor in Agronomy, plant ecology and rangeland management. He is the head of the Range Ecology Laboratory at the Institut des Régions Arides of Medenine (Tunisia). Contribution: Contributions to the idea and experimental design of this study.
Mounir Louhaichi PhD, Senior rangeland scientist at ICARDA. He is the team leader of the Rangeland Ecology and Management Unit. Contribution: Contributions to the idea and experimental design of this study; writing and interpretation of results and discussion and notable contribution to final revision.
Acknowledgements
We would like to thank the International Center for Agricultural Research in the Dry Areas (ICARDA), the CGIAR Research Program on Dryland Systems (CRP-DS), the CGIAR Research Program on Climate Change, Agriculture and Food Security (CCAFS) and the Arid Land Institute (IRA, Tunisia) for the co-financial support of this study.
References
- Angassa, A. 2014. “Effects of grazing intensity and bush encroachment on herbaceous species and rangeland condition in southern Ethiopia.” Land Degradation & Development 25: 438–451.
- Barbour, M. G., J. H. Burk, and W. D. Pitts. 1987. “Method of Sampling the Plant Community.” Chap. 9 in Terrestrial Plant Ecology. 2nd ed. Menlo Park, CA: Benjamin Cummings.
- Barrachina, M., J. Cristóbal, and A. F. Tulla. 2015. “Estimating above-ground biomass on mountain meadows and pastures through remote sensing.” International Journal of Applied Earth Observation and Geoinformation 38: 184–192.
- Bauer, Th, and P. Strauss. 2014. “A rule-based image analysis approach for calculating residues and vegetation cover under field conditions.” Catena 113: 363–369.
- Booth, D. T., S. E. Cox, C. Fifield, M. Phillips, and N. Williamson. 2005. “Image analysis compared with other methods for measuring ground cover.” Arid Land Research and Management 19: 91–100.
- Booth, D. T., S. E. Cox, M. Louhaichi, and E. J. Douglas. 2004. “Technical note: lightweight camera stand for close-to-earth remote sensing.” Journal of Range Management 57: 675–678.
- Booth, D. T., S. E. Cox, T. W. Meikle, and C. Fitzgerald. 2006. “The accuracy of ground-cover measurements.” Rangeland Ecology and Management 59: 179–188.
- Chaichi, M. R., M. M. Saravi, and A. Malekian. 2005. “Effects of Livestock Trampling on Soil Physical Properties and Vegetation Cover (Case Study: Lar Rangeland, Iran).” International Journal of Agriculture & Biology 7: 904–908.
- Cooper, W. S. 1924. “An apparatus for photographic recording of quadrats.” Journal of Ecology 12: 317–321.
- Cox, G. W. 1990. Laboratory Manual of General Ecology. 6th ed. Dubuque, Iowa: WMC Brown, Publishers.
- Frank, D. A., and S. J. McNaughton. 1990. “Aboveground biomass estimation with the canopy intercept method: a plant growth form caveat.” Oikos 57: 57–60.
- Gilbert, J. A., and K. R. Butt. 2009. “Evaluation of digital photography as a tool for field monitoring in potentially inhospitable environments.” Mires and Peat 5: 1–6.
- Hill, D., M. Fasham, G. Tucker, M. Shewry, and P. Shaw. 2005. Handbook of Biodiversity Methods: Survey. Evaluation and Monitoring. Cambridge: Cambridge University Press.
- Inoue, T., S. Nagai, H. Kobayashi, and H. Koizumi. 2015. “Utilization of ground-based digital photography for the evaluation of seasonal changes in the aboveground green biomass and foliage phenology in a grassland ecosystem.” Ecological Informatics 25: 1–9.
- Johnson, M. D., M. Louhaichi, D. E. Johnson, and A. Pearson. 2015. VegMeasure 2 Program. Version 2.2. Amman, Jordan: ICARDA.
- Laliberté, A. S., A. Rango, J. E. Herrick, E. L. Fredrickson, and L. Burkett. 2007. “An object-based image analysis approach for determining fractional cover of senescent and green vegetation with digital plot photography.” Journal of Arid Environments 69: 1–14.
- Larson, L. L., M. Louhaichi, P. E. Clark, and D. E. Johnson. 2013. “A Suggested Sampling Protocol for Sagebrush/Grassland Monitoring.” Paper presented at the 22nd International Grasslands Congress, Sydney, September 15–19.
- Lefsky, M. A., W. B. Cohen, D. J. Harding, G. G. Parker, S. A. Acker, and S. T. Gower. 2002. “Lidar remote sensing of above-ground biomass in three biomes.” Global Ecology and Biogeography 11 (5): 393–399.
- Louhaichi, M., M. M. Borman, and D. E. Johnson. 2001. “Spatially located platform and aerial photography for documentation of grazing impacts on wheat.” Geocarto International 16 (1): 65–70.
- Louhaichi, M., A. K. Salkini, and S. L. Petersen. 2009. “Effect of small ruminant grazing on the plant community characteristics of semiarid Mediterranean ecosystems.” International Journal of Agriculture & Biology 11: 681–689.
- Louhaichi, M., M. D. Johnson, P. E. Clark, and D. E. Johnson. 2012. “Developing a coherent monitoring system for Mediterranean grasslands.” In New Approaches for Grassland Research in a Context of Climatic and Socio-Economic Changes, edited by Z. Acar, A. Lopez-Francos, and C. Porqueddu. Options Méditerranéennes 102: 47–51.
- Louhaichi, M., D. E. Johnson, L. M. Richman, and M. Carpinelli. 2012b. “Native forbs response to herbicide applications in Eastern Oregon.” Australian Journal of Rangelands 34: 47–53.
- Louhaichi, M., M. D. Johnson, A. L. Woerz, A. W. Jasra, and D. E. Johnson. 2010. “Digital charting technique for monitoring rangeland vegetation cover at local scale.” International Journal of Agriculture & Biology 12: 406–410.
- Louhaichi, M., D. A. Pyke, S. E. Shaff, and D. E. Johnson. 2013. “Monitoring restoration impacts to endemic plant communities in soil inclusions of arid environments.” International Journal of Agriculture & Biology 15: 767–771.
- Louhaichi, M., F. Ghassali, A. K. Salkini, and S. L. Petersen. 2012. “Effect of sheep grazing on rangeland plant communities: Case study of landscape depressions within Syrian arid steppes.” Journal of Arid Environments 79: 101–106.
- Luo, S., C. Wang, F. Pan, X. Xi, G. Li, S. Nie, and S. Xia. 2015. “Estimation of wetland vegetation height and leaf area index using airborne laser scanning data.” Ecological Indicators 48: 550–559.
- Luscier, J. D., W. L. Thompson, J. M. Wilson, B. E. Gorham, and L. D. Dragut. 2006. “Using digital photographs and object-based image analysis to estimate percent ground cover in vegetation plots.” Frontiers in Ecology and the Environment 4: 408–413.
- Marsett, R. C., J. Qi, P. Heilman, H. Sharon, M. Biedenbender, C. Watson, S. Amer, M. Weltz, D. Goodrich, and R. Marsett. 2006. “Remote Sensing for Grassland Management in the Arid Southwest.” Rangeland Ecology and Management 59: 530–540.
- Mekuria, W., and E. Aynekulu. 2013. “Exclosure land management for restoration of the soils in degraded communal grazing lands in Northern Ethiopia.” Land Degradation & Development 24: 528–538.
- Mureithi, S. M., A. Verdoodt, J. T. Njoka, C. K. K. Gachene, F. Warinwa, and E. Van Ranst. 2014. “Impact of community conservation management on herbaceous layer and soil nutrients in a Kenyan semi-arid savannah.” Land Degradation & Development. doi:10.1002/ldr.2315.
- Olmstead, M. A., R. Wample, S. Greene, and J. Tarara. 2004. “Nondestructive measurement of vegetative cover using digital image analysis.” HortScience 39 (1): 55–59.
- Palacio, R. G., A. J. Bisigato, and P. J. Bouza. 2014. “Soil erosion in three grazed plant communities in northeastern Patagonia.” Land Degradation & Development 25: 594–603.
- Pelland, M., P. Shaver, D. A. Pyke, and J. E. Herrick. 2000. Interpreting indicators of rangeland health (version 3). Bureau of Land Management, United States, Department of Interior, Denver, CO: Technical Reference of the National Science and Technology Center.
- Preuss, C. P., M. Louhaichi, C. Y. Huang, and F. C. Ogbonnaya. 2012. “Genetic variation in the early vigor of twenty elite spring wheat under phosphate stress as characterized through digital charting.” Field Crops Research 127: 71–78.
- Radloff, F. G. T., and L. Mucina. 2007. “A quick and robust method for biomass estimation in structurally diverse vegetation.” Journal of Vegetation Science 18: 719–724.
- Scanlan, J. C., J. G. McIvor, S. G. Bray, R. A. Cowley, L. P. Hunt, L. I. Pahl, and G. L. Whish. 2014. “Resting pastures to improve land condition in northern Australia: guidelines based on the literature and simulation modelling.” The Rangeland Journal 36 (5): 429–443.
- VegMeasure. 2015. VegMeasure User Manual Series. Field manual. Volume 1. ICARDA (http://www.vegmeasure.org/ accessed 13 May 2015).
- Wiles, L. J. 2011. “Software to quantify and map vegetative cover in fallow fields for weed management decisions.” Computers and Electronics in Agriculture 78: 106–115.
- Wu, G. L., G. Z. Du, Z. H. Liu, and S. Thirgood. 2009. “Effect of fencing and grazing on a Kobresia-dominated meadow in the Qinghai-Tibetan Plateau.” Plant and Soil 319: 115–126.
- Wu, G. L., W. Li, X. P. Li, and Z. H. Shi. 2011. “Grazing as a mediator for maintenance of offspring diversity: Sexual and clonal recruitment in alpine grassland communities.” Flora 206: 241–245.