Abstract
There is an increasing interest in the taxonomy and distribution of the forms of the Portulaca oleracea complex. The information accruing from specimens collected in the Italian peninsula and surrounding islands (Sicily, Sardinia, Corsica and Malta) is here described. Eleven morphotypes were recorded: ‘P. cypria’, ‘P. granulatostellulata’, ‘P. nitida’, ‘P. oleracea’, ‘P. papillatostellulata’, ‘P. rausii’, ‘P. sardoa’, ‘P. sativa’, ‘P. sicula’, ‘P. trituberculata’, ‘P. zaffranii’ and a still unclear form Portulaca oleracea f. Three occur in almost all the Italian peninsula and adjacent islands; three are scattered in the Italian peninsula and in the adjacent islands; the remnant have a distribution restricted to the islands such as Sicily and Sardinia. The morphotypes can be divided into two main categories: seeds smooth, without ornamentations; seeds with ornamentations. The morphotypes with ornamentations are more widespread than smooth ones, probably because seed ornamentations play an important role in seed dispersal, which is perhaps mainly anthropochorous given that P. oleracea is a synanthropic species that can tolerate mechanical disturbance. There are cases of multiple occurrence, as evidenced by the presence of different morphotypes in some of the sites. Such populations can count up to five morphotypes growing together. Seven morphotypes were here recorded from Malta; they are all hexaploid, even those which in other areas are tetraploid.
Introduction
Portulaca oleracea L. (Portulacaceae), a herbaceous annual succulent plant, is a vigorous colonizer of disturbed and waste habitats (it can also be a weed in cultivated fields, see Tutin et al. Citation1993) in all continents from temperate to tropical zones. Such a wide distribution is due to its great adaptability (Zimmerman Citation1976; Matthews, Ketron, and Zane Citation1993) and to the high rate of seed production (Zimmerman Citation1976). Its geographical origin is uncertain (Ocampo and Columbus Citation2012): many hypotheses indicate different areas of the Old World, but it was also found in America in pre-Columbian times (Chapman, Stewart and Yarnell Citation1973; Byrne and McAndrews Citation1975). It is also cultivated as a vegetable in many parts of the world (Bel Hadj Salah and Chemli Citation2004). The species, which is mainly autogamous (Kim and Carr Citation1990; Danin, Domina, and Raimondo Citation2008; Tison and de Foucault Citation2014), is highly polymorphic, both in its habitus and in seed tegument ornamentation. Populations include prostrate, ascending or fully erect individuals (Danin et al. Citation2014) and the morphological variability is notable also among the populations, depending on trophic and ecological conditions (Bel Hadj Salah and Chemli Citation2004). Seed tegument is characterized by microsculptures (papillae, tubercles) and testa cells with different shape and ornamentation (stellulate, isodiametric or elongated, rich in microsculptures or flat). The chromosome number is also highly variable. Three main ploidy levels have been detected, with base chromosome number n = 9 (Matthews, Ketron, and Zane Citation1994), e.g. diploids are 2n = 18, tetraploids 2n = 36 and hexaploids 2n = 54 (Danin et al. Citation2012). Additional reports of pentaploid forms (2n = 45) are known from India (Sharma and Bhattacharyya Citation1956), and a case of chromosome number 2n = 52 was found in Japan (Sugiura Citation1936) and 2n = 48 was reported for P. sardoa (Danin et al. Citation2012). These genomic differences obviously represent a reproductive barrier among the different caryotypes (Danin and Reyes-Betancort Citation2006). The chromosome number, together with seed tegument ornamentations, was considered by several authors to be of taxonomic value, so that P. oleracea was regarded as an aggregate of 19 microspecies (Danin, Baker, and Baker Citation1978; Danin and Reyes-Betancort Citation2006; Danin, Domina, and Raimondo Citation2008; Danin Citation2011; Danin and Raus Citation2012; Danin et al. Citation2012). “Microspecies” is a term used to designate clonal individuals, genotypes or ecotypes that evolve separately from others because of their apomictic reproduction (Dickinson Citation1998; Pihu et al. Citation2009). However, for P. oleracea, an eventual apomixis has not been reported; indeed, the term “microspecies” was probably used in an inappropriate way for this species. Recent phylogenetic studies (Ocampo and Columbus Citation2012) show that P. oleracea is a polyphyletic species, with evidence that it is a polyploid complex (Soltis and Soltis Citation1999; Soltis, Soltis, and Tate Citation2003; Soltis et al. Citation2004). Hence, even though some authors have recently published important contributions (El-Bakatoushi et al. Citation2013; Walter, Vekslyarska, and Dobeš Citation2015), the taxonomy of P. oleracea is still open to debate. The various combinations of seed tegument, cell shape and cell ornamentations allow the recognition of different morphotypes, which are intended as informal infraspecific taxonomic categories based on precise morphological features (Maiti and Maiti Citation2011), without connexion with genome asset or reproductive strategies. Such morphotypes are constant within the individuals, so that the individuals themselves can be readily separated within the same population. To contribute to the debate in progress we focused our investigation on an area for which information on the infraspecific diversity of this species is fragmentary and irregular: the Italian peninsula and adjacent islands. We included in our study Corsica, the Maltese Islands and some small valleys of Switzerland in the Canton of Grisons, very close (2–3 km at most) to the Italian border, because all these areas are normally considered in the Italian floristic check lists (e.g. Zàngheri Citation1976; Pignatti Citation1982).
In Italy, the first taxonomic study of P. oleracea (in this case treated as an aggregate of microspecies) was performed by Ricceri and Arrigoni (Citation2000). They followed Danin, Baker, and Baker’s (Citation1978) system and reported the presence in Italy of five microspecies sensu Danin and Reyes-Betancort (Citation2006) and Danin, Domina, and Raimondo (Citation2008). The present article, summing up all the forms known so far (Danin and Raus Citation2012), considers P. oleracea as a polymorphic species according to Walter, Vekslyarska, and Dobeš (Citation2015). Previous studies on the variability of P. oleracea s.l. are available only for a few regions: Trentino-Alto Adige (Wilhalm et al. Citation2008), Emilia-Romagna (Danin et al. Citation2014), Sicily (Danin, Domina, and Raimondo Citation2008; Domina et al. Citation2013), Sardinia (Danin et al. Citation2012) and Corsica (Danin Citation2011). Some further data come from sporadic analyses presented in Domina, Schäfer, and Danin (Citation2010), Danin (Citation2011) and Danin and Raus (Citation2012). For many Italian regions and the Maltese archipelago this is the first in-depth investigation of the infraspecific diversity of P. oleracea s.l.; the study by Ricceri and Arrigoni (Citation2000) is only partially comparable to ours, due to their different opinions about the infraspecific taxonomy of this species and to the significantly lower number of samples examined.
This paper is a synthesis of all the available information concerning the presence and the distribution of the various morphotypes of P. oleracea s.l. in the Italian peninsula and in surrounding islands. It is important to consider as many sources of information as possible, also because of the peculiar geographical position and geomorphological conformation of the Italian peninsula and the surrounding islands, which is probably one of the reasons for the great variety of environments of the Italian territory.
The aims of the present research were:
| • | to define the pattern of the distribution, in the Italian peninsula and adjacent islands, of the morphotypes of P. oleracea, to provide a contribution for the assessment of their general distribution; | ||||
| • | to extend caryological systematic studies on P. oleracea complex to the Maltese Islands, which were not considered in previous researches (as shown by Walter, Vekslyarska, and Dobeš, Citation2015, the chromosome number can vary also in the same morphotype, so investigating an unexplored zone could reveal novelties). | ||||
Materials and methods
Seed samples analysis and distribution assessment
The definition of fine-scale patterns was based on information gathered from herbarium specimens, field collections and published data.
Classification was based on the analysis of seeds; for each morphotype we maintained the names formerly attributed to the microspecies, only posing them between inverted commas to avoid confusion with the microspecies mentioned in previous papers. The seed analysis was performed using a Reichert Austria No. 306 284 dissecting microscope at 40 × magnification. Identification was based on the general key for the P. oleracea complex provided by Danin and Raus (Citation2012), which takes into account the following morphological features:
| • | seed diameter | ||||
| • | shape of testa cells (digitated, elongated, isodiametric) | ||||
| • | surface of testa cells (smooth or with protuberances) | ||||
| • | type of protuberances (tubercles, papillae, granula) | ||||
| • | number and position of the protuberances in the testa cells. | ||||
Photographs of the seeds were taken using a NovaNanoSEM 450-FEI scanning electronic microscope.
As a whole, we considered for this study 902 samples (see Supplemental data online).
The samples analysed ex novo for this study are 347 and are divided into 105 samples from historical herbaria (early 1800 to 1950), which were all taken from herbarium specimens, and 242 samples from recent collections (1951–2013), which correspond either to specimens already inserted in herbarium sheets, or to field collections only sometimes accompanied by the herbarium specimens (the samples coming from a herbarium specimen are indicated with X in the Supplemental data online).
Published data (Danin, Domina, and Raimondo Citation2008; Danin Citation2011, Citation2012; Danin et al. Citation2012, Citation2014; Domina et al. Citation2013), which were verified and, whenever necessary, corrected, provided information on another 555 seed samples, eight of which came from historical herbaria (period 1880–1900) and the others collected in recent years.
Concerning the samples analysed ex novo, we proceeded in the following way. For the samples originating from historical herbaria, 1–50 seeds (depending on exsiccatum richness) were analysed from each specimen. When a sheet contained more than one individual, seeds were taken from each of them. For the recent samples, if they came from herbarium sheets, we proceeded as described above, but in the case of field collections (years 2006–2013) 1–10 individuals (depending on population size) were sampled at each site, taking five capsules for each plant, posing a distance of 1 m between two sampled plants (when individuals grew in small groups, all individuals were sampled).
Most of the samples were collected from 0 to 1100 m above sea level. The only exception is a sample (Leg. Vaccari, Wilyek et Mailuser, 1903, in FI) collected at 1700 m above sea level.
Using the results of the classification, a fine-scale pattern relative to Italy and adjacent islands was assessed for each morphotype and a classification of the distribution models was proposed and discussed. To better visualize the presence of each morphotype in the past and in the present, we drew a distribution map for each one, distinguishing historical herbarium data and present collections.
Due to the occasional occurrence of plants with different morphotypes in the same sampling site, the percentages of abundance of every morphotype were not calculated basing on the total number of samples, but on the total number of records (941), i.e. the sum of all the occurrences of the various morphotypes in the samples.
Furthermore, we grouped all the distinguished morphotypes into three main categories, depending on seed tegument ornamentations:
Type I seeds almost completely smooth, without ornamentations (e.g. ‘P. nitida’)
Type II seeds with up to two tubercles per cell, or with papillae or granula only (e.g. ‘P. cypria’)
Type III seeds with more than two tubercles per cell (e.g. ‘P. rausii’).
Caryological analysis
Caryological analyses were performed on root tips obtained from fresh material, considering all the morphotypes discovered in the Maltese Islands. Various subsamples of two to five seeds each (at least one subsample for each morphotype) were analysed. These investigations are aligned to those already performed for other large Mediterranean islands (Danin, Domina, and Raimondo Citation2008; Danin et al. Citation2012). We followed the method already tested by Danin et al. (Citation2012). The material was treated with 0.3% colchicine solution for 3 hours, and then fixed in a modified Carnoy solution (five parts of ethanol to two parts of glacial acetic acid) for 30 min. After rinsing in water, tissues were hydrolysed in 1 m HCl for 8 min at 60°C, placed in Schiff reagent for 30 min, and then squashed and stained in 50% acetic acid. Preparations were made permanent by freezing with carbon dioxide (CO2), dehydrating in ethanol and mounting in Canada balsam. Chromosomes were counted on mitotic metaphase plates under an Axiophot optic microscope at 1000 × magnifications.
Results
Seed samples analysis and distribution assessment
As a whole, 11 morphotypes have been recognized in the Italian peninsula and adjacent islands during the present study (Figures ): ‘P. cypria’ Danin (2n = 54), ‘P. granulatostellulata’ (Poelln.) C. Ricceri et P.V. Arrigoni (2n = 36), ‘P. nitida’ (Danin et H.G. Baker) C. Ricceri et P.V. Arrigoni (2n = 36), ‘P. oleracea’ L. (2n = 54), ‘P. papillatostellulata’ (Danin et H.G. Baker) Danin (2n = 54), ‘P. rausii’ Danin, Domina et Raimondo (2n = 54), ‘P. sardoa’ Danin, Bagella et Marrosu (2n = 48), ‘P. sativa’ Haw. (2n = 54), ‘P. sicula’ Danin, Domina et Raimondo (2n = 54), ‘P. trituberculata’ Danin, Domina et Raimondo (2n = 54), ‘P. zaffranii’ Danin (2n = 36). To these morphotypes, a twelfth form has to be added, present only in six samples from the Maltese archipelago, which seems to have intermediate morphological features. It has a chromosome number (see following paragraph Caryological analysis) similar to the hexaploids (2n = 54); however, seed size would be in accord with the ploidy level sensu Danin, Baker, and Baker (Citation1978). In view of this, we have provisionally referred these specimens to an as yet undefined Portulaca oleracea f. Further studies will be necessary to verify if it is a really new morphotype.
Figure 1. Synopsis of the morphotypes of Portulaca oleracea found in the Italian peninsula and surrounding islands. View of the whole seed: (A) ‘P. cypria’, (B) ‘P. granulatostellulata’, (C) ‘P. nitida’, (D) ‘P. oleracea’, (E) ‘P. papillatostellulata’, (F) ‘P. rausii’, (G) ‘P. sardoa’, (H) ‘P. sativa’, (I) ‘P. sicula’, (J) ‘P. trituberculata’, (K) ‘P. zaffranii’. Images (A) and (I) are from Danin, Domina, and Raimondo (Citation2008), modified; images (B), (C), (E) and (J) were taken by Dr Massimo Tonelli; the others are from A. Danin’s personal archive. For images (A) and (K), scale bar is 500 μm; for images (B), (D), (F), (G) and (I), scale bar is 200 μm; for images (C) and (E), scale bar is 300 μm; for image (H), scale bar is 1 mm; for image (J), scale bar is 400 μm.
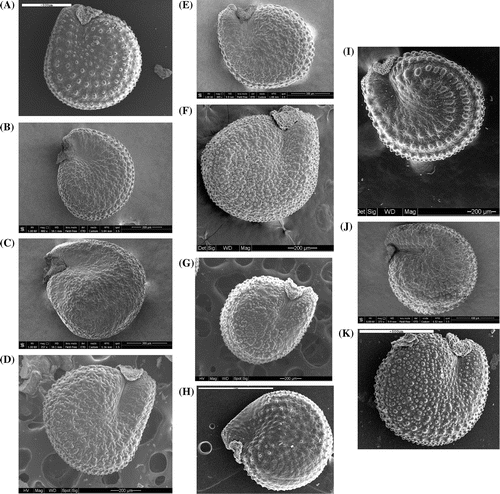
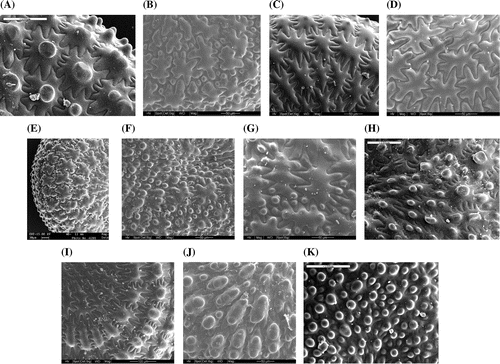
Figure 2. Synopsis of the morphotypes of Portulaca oleracea found in the Italian peninsula and surrounding islands. Close up of the lateral face of the seed: (A) ‘P. cypria’, (B) ‘P. granulatostellulata’, (C) ‘P. nitida’, (D) ‘P. oleracea’, (E) ‘P. papillatostellulata’, (F) ‘P. rausii’, (G) ‘P. sardoa’, (H) ‘P. sativa’, (I) ‘P. sicula’, (J) ‘P. trituberculata’, (K) ‘P. zaffranii’. Images (A) and (E) are from Danin and Raus (Citation2012), modified; image (H) is from Danin et al. (Citation2014), modified; the others are from A. Danin’s personal archive. For images (A), (H), (I) and (K), scale bar is 100 μm; for images (B), (C), (D), (F) and (J), scale bar is 50 μm; for images (E) and (G), scale bar is 30 μm.
Considering all 902 samplings, multiple occurrences were noted in 31 of them (3.4%). The most interesting case of multiple occurrence was from Sorrento (Naples), in two small public flowerbeds (each 1–2 m2), where five morphotypes were growing together (Leg. Danin, 18-12-2012).
The Italian peninsula is characterized (Figure ) by the presence of six morphotypes (here listed in alphabetical order): ‘P. cypria’, ‘P. granulatostellulata’, ‘P. nitida’, ‘P. oleracea’, ‘P. papillatostellulata’ and ‘P. trituberculata’. To these can be added ‘P. sativa’, which was found only in archaeological layers in Emilia-Romagna (northern Italy; see Bosi et al. Citation2009; Danin et al. Citation2014). In Sicily, we identified the six morphotypes listed for the Italian peninsula, plus ‘P. rausii’, ‘P. sicula’ and ‘P. zaffranii’. In Sardinia, we found five morphotypes already present in continental Italy (‘P. granulatostellulata’, ‘P. nitida’, ‘P. oleracea’, ‘P. papillatostellulata’, ‘P. trituberculata’), plus ‘P. sardoa’. In Corsica we found again six morphotypes, five of which present in peninsular Italy (‘P. cypria’, ‘P. granulatostellulata’, ‘P. oleracea’, ‘P. papillatostellulata’ and ‘P. trituberculata’), plus ‘P. sardoa’.
Figure 3. Distribution maps of the Portulaca oleracea morphotypes cited. Their presence is expressed as follows: pale grey, presence attested only in historical herbarium samples (before 1950); dark grey, presence attested both in historical herbarium samples and in recent collections; black, presence attested only in recent collections. (A) ‘P. cypria’, (B) ‘P. granulatostellulata’, (C) ‘P. nitida’, (D) ‘P. oleracea’, (E) ‘P. papillatostellulata’, (F) ‘P. rausii’, (G) ‘P. sardoa’, (H) ‘P. sicula’, (I) ‘P. trituberculata’, (J) ‘P. zaffranii’, (K) Portulaca oleracea f.
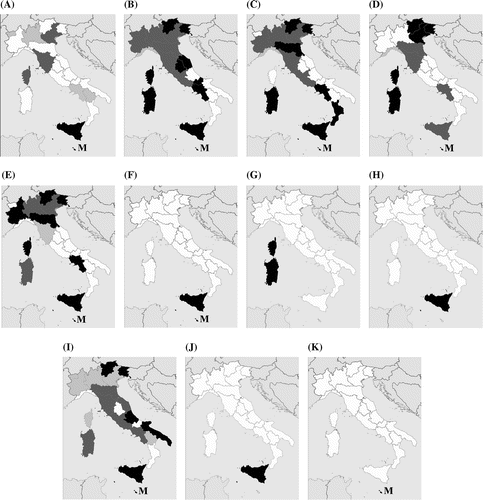
In the Maltese Islands we found the same six morphotypes as in the Italian peninsula, plus ‘P. rausii’ and the as yet undefined Portulaca oleracea f.
The distribution of several morphotypes (‘P. cypria’, ‘P. granulatostellulata’, ‘P. nitida’, ‘P. oleracea’, ‘P. papillatostellulata’ and ‘P. trituberculata’) differs in the past (pre-1950 samples) and in the present (samples collected in the years 1951–2013). In some cases, recent collections allowed confirmation of only part of the morphotypes already discovered in the historical herbarium specimens: for example, in Veneto one can find ‘P. cypria’, ‘P. granulatostellulata’, ‘P. nitida’, ‘P. oleracea’ and ‘P. papillatostellulata’ in recent collections, whereas in the historical herbarium samples ‘P. trituberculata’ is also present (Leg. Goiran, 1897, in FI; Leg. Minio, 1921, in FI – sp. pl. –) and ‘P. oleracea’ is lacking. In other instances, in contrast, recent collections allowed us to increase the number of morphotypes known for the region: in Campania for example only three forms can be detected from historical herbarium samples (‘P. cypria’, ‘P. oleracea’ and ‘P. trituberculata’; samples preserved in NAP), one of which (‘P. cypria’) is exclusive to the historical herbaria, whereas in recent collections (Leg. Danin, Citation2012 – sp. pl. –) one can find also ‘P. granulatostellulata’, ‘P. nitida’ and ‘P. papillatostellulata’. It has to be noted that in the historical herbarium samples some forms never appear (‘P. rausii’, ‘P. sardoa’, ‘P. sicula’ and ‘P. zaffranii’), even in the case of historical herbarium samples that came from the same regions where such morphotypes are present today.
Among the records originating from the historical herbaria, the most widespread morphotype was ‘P. trituberculata’ (37.7%), followed by ‘P. granulatostellulata’ (28.7%), ‘P. cypria’ (11.5%) and ‘P. nitida’ (10.7%). Among the records originating from recent collections (years 1951–2013), the most widespread morphotype was rather ‘P. granulatostellulata’ (33.6%), followed by ‘P. nitida’ (26.4%), ‘P. trituberculata’ (19.3%) and ‘P. papillatostellulata’ (7.7%).
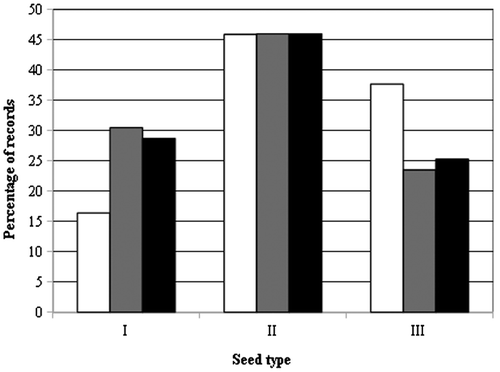
Concerning the three main categories established according to seed tegument ornamentations (see Materials and Methods), type I includes ‘P. nitida’ and ‘P. oleracea’, type II includes ‘P. cypria’, ‘P. granulatostellulata’, ‘P. papillatostellulata’, ‘P. sardoa’ and ‘P. sicula’, and type III includes ‘P. rausii’, ‘P. trituberculata’, ‘P. zaffranii’ and Portulaca oleracea f. If we correlate the number of records of the morphotypes with seed ornamentations, we can see (Figure ) that type II is the most widespread (45.9% considering only historical herbarium samples, 46% considering only recent collections, 46% taking into account all the samples examined for this work). Types I and III are clearly secondary: type I is present at 16.4, 30.5 and 28.7%, respectively, whereas type III is present at 37.7, 23.5 and 25.3%. Hence, we could hypothesize that the morphotypes that are rich in ornamentations (types II and III) are more widespread than the smooth ones.
Caryological analysis
Caryological analysis revealed that all the analysed samples from the Maltese Islands were hexaploid (2n = 54), even those ascribed to morphotypes that were previously (Danin, Baker, and Baker Citation1978) considered to have a different ploidy level (‘P. granulatostellulata’ and ‘P. nitida’). Some specimens were associated with seeds presenting different characteristics from those found in the already described morphotypes. Based on these findings, we referred these specimens to a generic Portulaca oleracea f., which description requires further studies. All the other specimens were referred to already described morphotypes.
Discussion
This study provides a comprehensive picture of the presence of all the morphotypes of P. oleracea so far known in Italy and in the adjacent islands. Our findings, although they are far from definitive for this subject, are a contribution to understand the distribution of such morphotypes in the investigated areas.
As a result of these findings, three patterns of distribution were recognized:
| • | presence in almost all the Italian peninsula and adjacent islands (‘P. granulatostellulata’, ‘P. nitida’, ‘P. trituberculata’); | ||||
| • | scattered occurrence in the Italian peninsula and in the adjacent islands (‘P. cypria’, ‘P. oleracea’, ‘P. papillatostellulata’); | ||||
| • | presence only in the islands (‘P. rausii’, ‘P. sardoa’, ‘P. sicula’, ‘P. zaffranii’, Portulaca oleracea f.). | ||||
The distribution of ‘P. sativa’ was not included in this classification because it was discovered only in two archaeological sites of Ferrara dated to the late fifteenth century, in a context of waste deposits of higher classes or even nobles (Bosi et al. Citation2009). At that time, it was probably cultivated as a vegetable. This hypothesis is supported by written Italian sources (Mattioli Citation1568; Felici Citation1572; Massonio Citation1627) and by the fact that it is still cultivated in some Mediterranean countries (Danin, Baker, and Baker Citation1978) and in other countries outside the Mediterranean area. In Italy its cultivation is only sporadic (at least in the last two centuries) and its tendency to scatter is probably scarce (Pignatti Citation1982; Picchi and Pieroni Citation2005).
In the following discussion of the patterns of distribution, we also integrated, when available, archaeobotanical data.
The first pattern concerns the group of the most widespread morphotypes. It should be noted that, in continental Italy, ‘P trituberculata’ has been present since the Roman period (Danin et al. Citation2014). ‘P. granulatostellulata’ is present in old herbarium samples, dating back to the nineteenth century: the first records are three samples, one from Abano, in Veneto (Leg. Felisi, 1842, in FER) and two from Ivrea, in Piedmont (Leg. Carestia, 30-7-1866, in FI; Leg. Carestia, 30-7-1866, in TO). This may suggest that its present distribution is the result of a long expansion phase. ‘P. nitida’, instead, seems to have expanded solely from the twentieth century onwards: the first record is a herbarium sample from the Capraia Island, in Tuscany (Leg. Sommier, 17-8-1896, in FI).
To the second pattern of distribution, less spread in the study area, are ascribed ‘P. cypria’, ‘P. oleracea’ and ‘P. papillatostellulata’. The presence of ‘P. cypria’ and ‘P. papillatostellulata’ is documented since the Roman age (Danin et al. Citation2014), whereas ‘P. oleracea’ appears for the first time in the first half of the nineteenth century (Leg. Gussone, period 1825-1860, in NAP; Leg. Sommier, 29-9-1850, in FI). At present, ‘P. papillatostellulata’ is not widespread; indeed, it appears to have been in decline already from the Roman period to the Middle Ages (Emilia-Romagna region; see Danin et al. Citation2014), so the current sporadic presence would seem to be the continuation of a historical regress. ‘P. cypria’, instead, which was sporadic in the Roman period and quite widespread during the Middle Ages and the Renaissance (Danin et al. Citation2014), is at present relatively infrequent, being limited (at least apparently) to Veneto, Tuscany, Sicily, Malta and Corsica. In any case, we have to remember that for this group of morphotypes, since the number of samples is not always comparable across the regions, particularly for the historical herbarium specimens, the data may be biased and our hypotheses have to be taken with caution.
To the third pattern of distribution are referred the morphotypes that are exclusive of insular regions, such as Sicily (‘P. rausii’, ‘P. sicula’ and ‘P. zaffranii’), Sardinia and Corsica (‘P. sardoa’) and Malta (P. oleracea f.). We have to remember that ‘P. rausii’ and ‘P. zaffranii’ are also present in other large Mediterranean islands, such as Crete, Cyprus or Rhodes (Danin, Domina, and Raimondo Citation2008), and in some countries facing the Mediterranean Sea (Euro+Med PlantBase Citation2006-onwards); in any case, in our study area they are found only in Sicily.
Based on our analyses, ‘P. granulatostellulata’, ‘P. nitida’ and ‘P. trituberculata’ would seem to be the most widespread forms in the study area. The first one, in particular, is one of the most widespread morphotypes even on a world scale (Danin, Baker, and Baker Citation1978). Such findings are consistent, in some respects, with what has been supposed for the P. oleracea complex in France (Tison and de Foucault Citation2014), where the most widespread forms would be ‘P. trituberculata’ in continental areas and ‘P. granulatostellulata’ in the Mediterranean ones. Our findings also confirm those of Danin (Citation2011), who remarked that, at least on a Euro-Mediterranean scale, the most widespread forms seem to be ‘P. granulatostellulata’ and ‘P. trituberculata’. Similar results were obtained in the analysis of the Maltese collections: the most abundant morphotype seems to be ‘P. trituberculata’, which represents 41.9% of the samples. However, the knowledge about the frequency of the various forms is still partially incomplete, despite the many hundreds of populations investigated in continental Europe and the Mediterranean basin (Danin Citation2011). Among the samples originating from the historical herbaria, the most common morphotypes are always ‘P. granulatostellulata’ and ‘P. trituberculata’, whereas ‘P. nitida’ is quite common only in the recent samples. The latter form could have experienced a broader diffusion only in the recent decades, although its abundance could also be explained by the great quantity of this morphotype registered in Sardinia.
The differences at a regional level are sometimes contrasting: increase (e.g. Corsica) or decrease (e.g. Lombardy, Tuscany) in the number of morphotypes, even when the number of the historical herbarium samples and the number of the recent ones are comparable. For the latter case, one can hypothesize a possible decrease in the infraspecific diversity, which would have occurred in the period after World War II and would be likely attributable to the well-known progressive environmental deterioration and homogenization provoked by man’s activities. Globally, there is also an increase of diffusion for ‘P. granulatostellulata’, ‘P. nitida’, ‘P. oleracea’ and ‘P. papillatostellulata’: the first one, for example, was recorded in eight Italian regions considering only the historical herbarium samples, whereas if we take into account the recent collections the regions of presence amount now to 17. However, we have to observe that the global number of records originating from historical herbarium samples is only 122, whereas the number of records of the recent collections is 819: such a difference would probably be a good explanation for the apparent diverse diffusion of the four above-mentioned morphotypes. In any case, caution is needed in considering the results of such a comparison, because the historical herbarium samples are about one-seventh of the samples originating from recent collections.
The most interesting aspect of such a comparison concerns the three main categories established based on seed tegument ornamentations. If we observe the distribution of the morphotypes in the past (samples before 1950) and in the present, we can see that there is a clear dominance of the forms with more ornamentations. A possible explanation may be found in the ecology of P. oleracea s.l.: a ruderal synanthropic species that tolerates mechanical disturbance. It is not unlikely that anthropogenic disturbance can be a dispersal vector (dissemination is probably mainly zoochorous and anthropochorous; see Miyanishi and Cavers Citation1980). Seeds with ornamentations could probably adhere better than the smooth ones to clothes, shoes or mud on shoes, so that they probably present a competitive advantage for dispersal. The best characteristics for the anthropochorous dispersal are indeed:
| • | seeds with small dimensions and little weight: it has been discovered that seeds with an average width of 2 mm, although they are smooth, could remain attached to mud on shoes for several kilometres (Wichmann et al. Citation2009). A weight of 10 mg, independently of tegument morphology, is the limit above which seeds do not attach to cattle hair or clothes (Römermann, Tackenberg, and Poschlod Citation2005); | ||||
| • | seed tegument with ornamentations and sculptures, even modest, on condition that they project out of the seed outline (see Römermann, Tackenberg, and Poschlod Citation2005). | ||||
Portulaca oleracea s.l. shows exactly these features, both in seed dimensions and weight and in tegument microsculptures: seeds are ~1 mm wide, weigh on average 0.1 mg (Friess and Maillet Citation1995) and, in most cases, are provided with ornamentations that are similar, in some regards, on a minor scale, to the appendages that project out of the seed silhouette. Furthermore, this species is normally prostrate and creeping and, because of its life in hostile and disturbed environments, it is quite natural that it is adapted to the anthropochorous dispersal. Human transportation is an excellent vehicle for the seeds to be brought over long distances away from the parent plant (Cousens, Dytham, and Law Citation2008, 70–73). It is presumable that the above-mentioned features allow a more efficient dispersal in the forms with seed ornamentations, and this seems to be confirmed by the notable difference that exists between the records of smooth forms (16.4% considering only herbarium samples, 30.5% considering only recent collections, 28.7% taking into account all the samples examined for this work) and those of forms with microsculptures in seed tegument (83.6, 69.5 and 71.3%, respectively). The only case of a smooth form that is relatively widespread is ‘P. nitida’, which seems to be quite abundant only in Sardinia (43.6% of the recent samples originating from that region). This datum probably explains the frequencies of the smooth seeds in the recent samples (see Figure ). Such extraordinary abundance, which perhaps is unique for the whole investigated area, might be due to a lesser human presence on the territory: Sardinia is among the least densely populated Italian regions (average density 69 inhabitants/km2, versus 201 inhabitants/km2 average for the entire Italy). Hence, ‘P. nitida’ perhaps represents a form adapted to dissemination in areas where anthropic disturbance is globally inferior to that of other regions, especially continental ones. Another possibility could be an as yet unclear adaptation to specific environmental characteristics, which would require a dedicated study.
Concerning the caryological analyses, the investigations performed in Sicily (Danin, Domina, and Raimondo Citation2008) and in Sardinia (Danin et al. Citation2012) seemed to confirm a constancy of genome and phenotype. In contrast, the Maltese specimens of our study show a constancy only of the caryotype, which is always hexaploid. This fact is proof that P. oleracea s.l. can produce identical morphotypes despite the different chromosome number: in other words, no correlation seems to exist between caryology and morphology, as already suspected by Danin and Raus (Citation2012) and later asserted by Walter, Vekslyarska, and Dobeš (Citation2015). Given the distribution of many morphotypes, which are today considered to be exclusive to one or two particular islands (e.g. ‘P. sardoa’, ‘P. sicula’), one could even think of a possible relation between caryotype and colonization of new areas, which would have perhaps influenced morphotype and phenotype. The possible reasons for such phenotypic differences can be very diverse, both genetic and ecological. For example, it is known that a sudden environmental variation is a stimulus to rapid genomic evolution (Stuessy and Crawford Citation1998): it would not be strange if such differentiation would be manifested also at the phenotype level. As already suggested by Danin, Domina, and Raimondo (Citation2008), it is likely that the differences in the vegetative characters may be linked to environmental variations. An analogous supposition can be made for seed morphology, which was always observed to remain constant, not only in the cultivation experiment under controlled conditions performed by Danin, Domina, and Raimondo (Citation2008), but also in natural conditions (Danin, Baker, and Baker Citation1978; Danin and Reyes-Betancort Citation2006). Similar considerations were also proposed by Wyatt (Citation1984), who analysed disjunct populations of Arenaria uniflora Muhl. and discovered a significant correlation between seed tegument morphology and environmental conditions, whereas, for the genus Brassica, Koul, Nagpal, and Raina (Citation2000) found that seed tegument features do not always closely reflect the genetic characteristics. If such considerations were valid also in the case of P. oleracea s.l., the Maltese unclear form (P. oleracea f.) could be a secondary innovation after a colonization event; in this case, the caryological analyses would corroborate, at least apparently, the hypothesis of the major diffusion of the morphotypes with ornamentations, given the higher adherence capacity, which is probably favoured in the sculptured forms. It has been noted (Danin and Raus Citation2012) that P. oleracea s.l. produces similar morphotypes with different chromosome numbers in various parts of the world: this may support both the idea of a rapid genomic evolution as a response to sudden environment variations, and the idea of a different phenotype as a response to environment conditions differing from the initial ones. The two aspects of the issue are complementary and not contradictory. In autogamous species, interpopulation morphological variability is a proxy of different environmental conditions (Allard Citation1988), although the cases of multiple occurrence in the same location would support the hypothesis that seed coat differences are mostly due to genetic differences (otherwise, one should have to think of a sort of hypersensibility to microenvironmental and trophic conditions, which would be quite strange in a ruderal species like P. oleracea s.l.). Furthermore, in some cases of multiple occurrence, the chromosome number of the morphotypes identified can vary, with tetraploid and hexaploid individuals in the same population. This fact, together with the different chromosome numbers in the same morphotype in different areas of the world, seems to show that P. oleracea s.l. may be an unstable species from a genetic viewpoint, which could be explained as a result of hybridization between different caryotypes or a product of an apomixis, although this has not yet been detected in P. oleracea s.l. (see for a comparison Diana Citation1992; Arrigoni and Diana Citation1993; Słomka, Wolny, and Kuta Citation2014). Such genetic instability is probably responsible for the high adaptability of the species, a likely hypothesis given the species’ ecology. A similar caryological variability within and among populations has been recently observed in Viola tricolor L., another ruderal and very adaptable species, as a reaction to soils polluted with heavy metals (Słomka, Siwińska, et al. Citation2011; Słomka, Sutkowska et al. Citation2011). To summarize, there is no single possible explanation for the variability of the seed coating micromorphology in P. oleracea s.l., at a caryotype and phenotype level.
Conclusions
The number of morphotypes detected in the studied territory is high, compared with the number of morphotypes so far known worldwide (19). This is a confirmation of the great biological value of the Mediterranean basin, which is known to be one of the “hot spots” of biodiversity of the world (Myers et al. Citation2000). The analysis of the historical herbarium samples, which were formerly determined simply as Portulaca oleracea L., allowed not only to identify the corresponding specimens in a more precise way, but also to discover some forms that were not known for certain regions, such as ‘P. oleracea’ and ‘P. papillatostellulata’ in Sardinia, discovered in herbarium samples dated at the 1970s (Leg. Arrigoni et Ricceri, 1971, in FI; Leg. Ricceri, 1971, in FI). In some cases, herbarium analyses allowed us to increment the number of morphotypes known for some regions: this was the case, for example, for Trentino-Alto Adige, Sardinia and Corsica. Furthermore, this study provided for various regions a discrete knowledge of the infraspecific diversity of P. oleracea, which was previously not documented in many cases. Nevertheless, further investigations are needed to better understand the distribution and, overall, the abundance of the different morphotypes. At the present state of the research, it seems that the most widespread morphotypes may be ‘P. granulatostellulata’, ‘P. nitida’ and ‘P. trituberculata’, which would be also the most widespread on a global scale. Furthermore, the discovery of a new, unclear form of P. oleracea s.l. in the Maltese archipelago suggests that much still remains to be learnt about the morphological variability of P. oleracea in its distribution range. For this reason, it is highly necessary to continue the investigations, to completely understand the infraspecific variability of this species and the geographical distribution of its forms, on a local and global scale.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was financed by academic institutional funds of the Università degli Studi di Modena e Reggio Emilia (person in charge Prof. M. Bandini Mazzanti).
Notes on contributors
A. Danin is a botanist, an Emeritus Professor in the Department of Ecology, Evolution, and Behaviour in the Life Science Institute of the Hebrew University of Jerusalem, Israel. Contribution: samples determination, data interpretation, coordination of the research.
F. Buldrini collaborates in various researches carried out by the Botanical Garden of Modena, mainly concerning plant systematics and plant diversity conservation. Contribution: samples collection, data elaboration, coordination of the research.
M. Bandini Mazzanti is Associate Professor of Systematic Botany at the University of Modena and Reggio Emilia. Her research activities concern archaeobotany and palynology. Contribution: data interpretation, critical reading of the manuscript.
G. Bosi is a researcher in Applied Botany and Museology at the University of Modena and Reggio Emilia. Her research activities concern archaeobotany, with a special attention to seeds and fruits. Contribution: data interpretation, critical reading of the manuscript.
M.C. Caria is a researcher at the Department of Science for Nature and Environmental Resources (University of Sassari). Her scientific research is aimed at knowledge of biodiversity and issues relative to habitat and species conservation. Contribution: caryological analysis, data interpretation.
D. Dandria is an entomologist interested in Maltese faunistics and floristics. He is semi-retired and is currently Museum Curator at the Department of Biology, University of Malta. Contribution: samples collection, critical reading of the manuscript.
E. Lanfranco is a botanist, currently semi-retired, and lecturer at the University of Malta. Contribution: samples collection, data interpretation, critical reading of the manuscript.
S. Mifsud is an active private plant researcher and taxonomist who has contributed to the flora of Malta for more than a decade with about 30 peer-reviewed botanical papers. Contribution: samples collection, data interpretation, critical reading of the manuscript.
S. Bagella is researcher at the University of Sassari. Her research activities concern Mediterranean flora and vegetation and conservation biology. Contribution: caryological analysis, data interpretation.
Supplemental data
The supplementary material for this paper is available online at http://dx.doi.org/10.1080/23818107.2016.1200482.
Supplemental_Online_Material.xls
Download MS Excel (157 KB)Acknowledgements
We thank all people who kindly gave us information or material for this study. In detail: Lucia Amadei, Museo Botanico Pisano, Dipartimento di Biologia, Università degli Studi di Pisa; Nicola M.G. Ardenghi, Herbarium Universitatis Ticinensis, Dipartimento di Scienze della Terra e dell’Ambiente, Università degli Studi di Pavia; Carlo Argenti, via Pietriboni 7, 32100 Belluno; Giovanna Becca, Dipartimento di Scienze della Natura e del Territorio, Università degli Studi di Sassari; Ilaria Bonini, Herbarium Universitatis Senensis, Siena; Maurizio Bovio, Museo Regionale di Scienze Naturali della Valle d’Aosta, Aosta; Massimo Buccheri, Sezione Botanica del Museo Friulano di Storia Naturale, Udine; Manuela De Matteis Tortora, Orto Botanico di Napoli; Gabriele Galasso, Sezione di Botanica, Museo Civico di Storia Naturale di Milano; Laurent Gautier, Conservatoire et Jardin Botaniques de la Ville de Genève; Laura Guglielmone, Dipartimento di Scienze della Vita e Biologia dei Sistemi, Università degli Studi di Torino; Domenico Lucarini, Polo Museale di Ateneo ed Orto Botanico, Università degli Studi di Camerino; Annalisa Managlia, Erbario dell’Università di Bologna; Chiara Nepi, Herbarium Centrale Italicum, Firenze; Simonetta Peccenini, DISTAV, Università degli Studi di Genova; Mauro Pellizzari, Gruppo Flora Ferrarese; Filippo Piccoli, Orto Botanico di Ferrara; Filippo Prosser, Museo Civico di Rovereto; Giovanni Repetto, Museo Civico “Federico Eusebio”, Alba; Maria Tavano, Museo Civico di Storia Naturale “Giacomo Doria”, Genova; Roberta Vallariello, Erbario dell’Orto Botanico di Napoli; Roberto Venanzoni, Dipartimento di Biologia Applicata, Università degli Studi di Perugia; Marisa Vidali, Dipartimento di Scienze della Vita, Università degli Studi di Trieste; and Thomas Wilhalm, Museo di Scienze Naturali dell’Alto Adige, Bolzano. Dr Liran Carmel and Prof. Joseph Hirschberg (Department of Genetics, The Alexander Silberman Life Sciences Institute, The Hebrew University of Jerusalem, Israel) and Prof. Lucia Conte (Dipartimento di Scienze Biologiche, Geologiche e Ambientali, Università degli Studi di Bologna) critically read the manuscript. Dr Massimo Tonelli (Centro Interdipartimentale per i Grandi Strumenti, Università degli Studi di Modena e Reggio Emilia) helped us to take some of the scanning electron micrographs of the seeds. Finally, we thank two anonymous reviewers, whose comments allowed us to greatly improve our manuscript.
References
- Allard, R. W. 1988. “Genetic changes associated with the evolution of adaptedness in cultivated plants and their wild progenitors.” Journal of Heredity 79 (4): 225–238.
- Arrigoni, P. V., and S. Diana. 1993. “Contribution à la connaissance du genre Limonium en Corse.” Candollea 48 (2): 638–677.
- Bel Hadj Salah, K., and R. Chemli. 2004. “Variabilité phénotypique de quelques populations de Pourpier (Portulaca oleracea L.) en Tunisie.” Acta Botanica Gallica: Botany Letters 151 (1): 111–119. doi:10.1080/12538078.2004.10516024.
- Bosi, G., P. M. Guarrera, R. Rinaldi, and M. Bandini Mazzanti. 2009. “Ethnobotany of purslane (Portulaca oleracea L.) in Italy and morfo-biometric analyses of seeds from archaeological sites of Emilia Romagna (Northern Italy).” In Plants and Culture: seeds of the cultural heritage of Europe, edited by Jean Paul Morel and Anna Maria Mercuri, 129–139. EdiPuglia: Bari. Available from http://www.plants-culture.unimore.it/book/11%20Bosi%20et%20alii.pdf
- Byrne, R., and J. H. McAndrews. 1975. “Pre-Columbian purslane (Portulaca oleracea L.) in the New World.” Nature 253: 726–727. doi:10.1038/253726a0.
- Chapman, J., R. B. Stewart, and R. A. Yarnell. 1973. “Archaeological evidence for precolumbian introduction of Portulaca oleracea and Mollugo verticillata in Eastern North America.” Economic Botany 28 (4): 411–412.
- Cousens, R., C. Dytham, and R. Law. 2008. Dispersal in Plants – A Population Perspective, 70–73. New York, NY: Oxford University Press.
- Danin, A. 2011. “Portulacaceae”. In Euro+Med Notulae, 5 - Notulae ad floram euro-mediterranean pertinentes No. 27, edited by W. Greuter, and E. von Raab-Straube. Willdenowia 41: 131–134.
- Danin, A. 2012. “Portulacaceae”. In Med-Checklist Notulae, 31, edited by W. Greuter and Th. Raus. Willdenowia 42 (2): 291.
- Danin, A., I. Baker, and H. G. Baker. 1978. “Cytogeography and taxonomy of the Portulaca oleracea L. polyploid complex.” Israel Journal of Botany 27: 177–211.
- Danin, A., F. Buldrini, M. Bandini Mazzanti, and G. Bosi. 2014. “The history of the Portulaca oleracea aggregate in the Emilia-Romagna Po Plain (Italy) from the Roman age to the present.” Plant Biosystems 148 (4): 622–634. doi:10.1080/11263504.2013.788098.
- Danin, A., M. C. Caria, G. M. Marrosu, and S. Bagella. 2012. “A new species of Portulaca oleracea aggregate from Sardinia, Italy.” Plant Biosystems 146 (1): 137–141. doi:10.1080/11263504.2012.681319.
- Danin, A., G. Domina, and F. M. Raimondo. 2008. “Microspecies of the Portulaca oleracea aggregate found on major Mediterranean islands (Sicily, Cyprus, Crete, Rhodes).” Flora Mediterranea 18: 89–107.
- Danin, A., and Th. Raus. 2012. “A key to 19 microspecies of the Portulaca oleracea aggregate.” In Proceedings of the Symposium Caryophyllales, edited by A. K. Timonin, A. P. Sukhorukov, G. H. Harper, and M. V. Nilova: 70–83. Moscow: M.V. Lomonosov State University.
- Danin, A., and J. A. Reyes-Betancort. 2006. “The status of Portulaca oleracea L. in Tenerife, the Canary Islands.” Lagascalia 26: 71–81.
- Diana, S. 1992. “Alcuni aspetti della biologia della riproduzione del genere Limonium.” [Some aspects of the reproduction biology of the genus Limonium] Giornale Botanico Italiano 126 (2): 187–195.
- Dickinson, T. A. 1998. “Taxonomy of agamic complexes in plants: a role for metapopulation thinking.” Folia Geobotanica 33: 327–332. doi:10.1007/BF03216208.
- Domina, G., P. A. Schäfer, and A. Danin. 2010. “Typification and taxonomic status of Portulaca macrantha (Portulacaceae).” Flora Mediterranea 20: 187–191.
- Domina, G., A. Soldano, F. Scafidi, and A. Danin. 2013. “Su alcune nuove piante delle Isole Pelagie (Stretto di Sicilia).” [On some new plants of the Pelagian Islands (Strait of Sicily)] Quaderni di Botanica Ambientale e Applicata 23: 41–44.
- El-Bakatoushi, R., A. M. Alframawy, M. Samer, L. El-Sadek, and W. Botros. 2013. “Evolution of the Portulaca oleracea L. aggregate in Egypt on molecular and phenotypic levels revealed by morphological, inter-simple sequence repeat (ISSR) and 18S rDNA gene sequence markers.” Flora 208: 464–477. doi:10.1016/j.flora.2013.07.008.
- Euro+Med PlantBase. 2006–onwards. Euro+Med PlantBase – the information resource for Euro-Mediterranean plant diversity. Published on the Internet http://ww2.bgbm.org/EuroPlusMed/ [accessed 4-6-2016].
- Felici, C. 1572. Lettera sulle insalate [Letter on the salads]. Bologna, Biblioteca Universale, Fondo Aldrovandi, ms. 78², II, cc. 1r–24v.
- Friess, N., and J. Maillet. 1995. “Influence of cucumber mosaic virus infection on the intraspecific competitive ability and fitness of purslane (Portulaca oleracea).” New Phytologist 132: 103–111. doi:10.1111/j.1469-8137.1996.tb04514.x.
- Kim, I. S., and G. D. Carr. 1990. “Reproductive biology and uniform culture of Portulaca in Hawaii.” Pacific Science 44: 123–129.
- Koul, R. R., R. Nagpal, and S. N. Raina. 2000. “Seed Coat Microsculpturing in Brassica and Allied Genera (Subtribes Brassicinae, Raphaninae, Moricandiinae).” Annals of Botany 86: 385–397. doi:10.1006/anbo.2000.1197.
- Maiti, P. K., and P. Maiti. 2011. Biodiversity: perception, peril, and preservation, 23. Delhi: PHI Learning.
- Massonio, S. 1627. Archidipno, o vero dell’insalata e dell’vso di essa. Trattato nuouo, curioso, e non mai più dato in luce; da Salvatore Massonio Scritto, e diuiso in Sessanta otto Capi; Dedicato a’ molto Illustri Signori fratelli Lvdovico, Antonio, e Fabritio Col’antonii [Archidipno, or on the salad and its use. New, curious and no longer edited treatise, written by Salvatore Massonio and divided into sixty-eight chapters, dedicated to the very illustrious Misters brothers Ludovico, Antonio and Fabrizio Colantonii]. In Venetia, Alessandro Maganza.
- Matthews, J. F., D. W. Ketron, and S. F. Zane. 1993. “The biology and taxonomy of the Portulaca oleracea L. (Portulacaceae) complex in North America.” Rhodora 95: 166–183.
- Matthews, J. F., D. W. Ketron, and S. F. Zane. 1994. “The seed surface morphology and cytology of six species of Portulaca (Portulacaceae).” Castanea 59 (4): 331–337.
- Mattioli, P. A. 1568. I Discorsi di M. Pietro Andrea Matthioli sanese, medico cesareo, et del Serenissimo Principe Ferdinando Archiduca d’Austria &c. Nelli sei libri Di Pedacio Dioscoride Anazarbeo della materia Medicinale. Hora di nuouo dal suo istesso autore ricorretti, & in più di mille luoghi aumentati. Con le figure grandi tutte di nuouo rifatte, & tirate dalle naturali & uiue piante, & animali, & in numero molto maggiore che le altre per auanti stampate. Con due Tauole copiosissime spettanti l’una à ciò, che in tutta l’opera si contiene, & l’altra alla cura di tutte le infirmità del corpo humano. Con priuilegio del Sommo Pontefice, della Illustrissima Signoria di Venetia, & di altri Principi. In Venetia, Appresso Vincenzo Valgrisi.
- Miyanishi, K., and P. B. Cavers. 1980. “The biology of Canadian weeds. 40, Portulaca oleracea L.” Canadian Journal of Plant Science 60: 953–963.
- Myers, N., R. A. Mittermeier, C. G. Mittermeier, G. A. B. da Fonseca, and J. Kent. 2000. “Biodiversity hotspots for conservation priorities.” Nature 403: 853–858. doi:10.1038/35002501.
- Ocampo, G., and J. T. Columbus. 2012. “Molecular phylogenetics, historical biogeography, and chromosome number evolution of Portulaca (Portulacaceae).” Molecular Phylogenetics and Evolution 63: 97–112. doi:10.1016/j.ympev.2011.12.017.
- Picchi, G., and A. Pieroni. 2005. Atlante dei prodotti tipici. Le Erbe [Atlas of the typical products. The herbs]. Roma: INSOR – Istituto Nazionale di Sociologia Rurale. RAI-AGRA, p. 317.
- Pignatti, S. 1982. Flora d’Italia. Bologna: Edagricole.
- Pihu, S., J. Hõimra, E. Köster, and M. Pärtel. 2009. “Environmentally Dependent Morphological Variability in Seven Apomictic Microspecies from Alchemilla L. (Rosaceae).” Folia Geobotanica 44: 159–176. doi:10.1007/s12224-009-9034-0.
- Ricceri, C., and P. V. Arrigoni. 2000. “L’aggregato di Portulaca oleracea L. (Portulacaceae) in Italia” [The aggregate of P. oleracea in Italy]. Parlatorea IV: 91–97.
- Römermann, C., O. Tackenberg, and P. Poschlod. 2005. “How to predict attachment potential of seeds to sheep and cattle coat from simple morphological traits.” Oikos 110 (2): 219–230.
- Sharma, A. K., and N. K. Bhattacharyya. 1956. “Cytogenetics of some members of Portulacaceae and related families.” Caryologia 8: 257–274.
- Słomka, A., D. Siwińska, E. Wolny, K. Kellner, and E. Kuta. 2011. “Influence of a Heavy-Metal-Polluted Environment on Viola tricolor Genome Size and Chromosome Number.” Acta Biologica Cracoviensia, Series Botanica 53 (1): 7–15.
- Słomka, A., A. Sutkowska, M. Szczepaniak, P. Malec, J. Mitka, and E. Kuta. 2011. “Increased genetic diversity of Viola tricolor L. (Violaceae) in metal-polluted enviroments.” Chemosphere 83: 435–442. doi:10.1016/j.chemosphere.2010.12.081.
- Słomka, A., E. Wolny, and E. Kuta. 2014. “Viola tricolor (Violaceae) is a karyologically unstable species.” Plant Biosystems 148 (4): 602–608. doi:10.1080/11263504.2013.788576.
- Soltis, D. E., and P. S. Soltis. 1999. “Polyploidy: recurrent formation and genome evolution.” Trends in Ecology & Evolution 14 (9): 348–352.
- Soltis, D. E., P. S. Soltis, and J. A. Tate. 2003. “Advances in the study of polyploidy since Plant Speciation.” New Phytologist 161: 173–191. doi:10.1046/j.1469-8137.2003.00948.x.
- Soltis, D. E., P. S. Soltis, J. C. Pires, A. Kovarik, J. A. Tate, and E. Mavrodiev. 2004. “Recent and recurrent polyploidy in Tragopogon (Asteraceae): cytogenetic, genomic and genetic comparisons.” Biological Journal of the Linnean Society 82 (4): 485–501. doi:10.1111/j.1095-8312.2004.00335.x.
- Stuessy, T. F., and D. J. Crawford. 1998. “Chromosomal stasis during speciation in angiosperms of oceanic islands.” In Evolution and Speciation of Island Plants, edited by T. F. Stuessy and M. Ono, 307–324. New York, NY: Cambridge University Press.
- Sugiura, T. A. 1936. “A list of chromosome numbers in angiospermic plants. II.” Proceedings of the Imperial Academy, Tokyo, 12: 144.
- Tutin, T. G., N. A. Burges, A. O. Chater, J. M. Edmondson, V. H. Heywood, D. M. Moore, D. H. Valentine, S. M. Walters, and D. A. Webb, eds. 1993. Flora Europaea. Second Edition. Cambridge: University Press, vol 1, 137–138.
- Tison, J.-M., B. de Foucault (coords.). 2014. Flora Gallica. Flore de France. Mèze: Biotope, p. 925.
- Walter, J., T. Vekslyarska, and C. Dobeš. 2015. “Flow cytometric, chromosomal and morphological analyses challenge current taxonomic concepts in the Portulaca oleracea complex (Portulacaceae, Caryophyllales.” Botanical Journal of the Linnean Society 179: 144–156. doi:10.1111/boj.12309.
- Wichmann, M. C., M. J. Alexander, M. B. Soons, S. Galsworthy, L. Dunne, R. Gould, C. Fairfax, M. Niggemann, R. S. Hails, and J. M. Bullocks. 2009. “Human-mediated dispersal of seeds over long distances.” Proceedings of the Royal Society B 276: 523–532. doi:10.1098/rspb.2008.1131.
- Wilhalm, T., W. Tratter, E. Schneider-Fürchau, H. Wirth, and C. Argenti. 2008. “Ergänzungen und Korrekturen zum Katalog des Gefäßpflanzen Südtirols (2).” [Additions and corrections to the catalog of the vascular plants of South Tyrol] Gredleriana 8: 615–626.
- Wyatt, R. 1984. “Intraspecific Variations in Seed Morphology of Arenaria uniflora (Caryophyllaceae).” Systematic Botany 9 (4): 423–431.
- Zàngheri, P. 1976. Flora Italica. Padova, Italy: CEDAM.
- Zimmerman, C. A. 1976. “Growth characteristics of weediness in Portulaca oleracea L.” Ecology 57 (5): 964–974.