Abstract
This paper presents notes on new records and the taxonomy of 12 vascular plant species from several continental African countries and Madagascar. Datura wrightii (Solanaceae) is a new species for Africa reported from Morocco. Amaranthus muricatus (Amaranthaceae) is reported for the first time in Tunisia, Barleria oenotheroides (Acanthaceae) – from Central African Republic and Senegal, Burkea africana (Fabaceae) – from Guinea-Bissau, Caylusea abyssinica (Resedaceae) – from Zambia, Digitaria ciliaris (Poaceae) – from Morocco. New data on distribution in Africa is provided for Camptorrhiza strumosa (Colchicaceae) and four Resedaceae taxa: Caylusea hexagyna, Ochradenus baccatus, Reseda arabica and Reseda villosa. The taxonomy of the enigmatic Madagascan endemic Mollugo caespitosa (Molluginaceae) is reviewed, and it is synonymized with Mollugo decandra. The lectotypes are designated for both Mollugo species.
Introduction
In the present article we provide new or revised records of nine native species (Barleria oenotheroides, Burkea africana, Camptorrhiza strumosa, Caylusea abyssinica, Caylusea hexagyna, Digitaria ciliaris, Ochradenus baccatus, Reseda arabica, Reseda villosa) and two alien species (Datura wrightii and Amaranthus muricatus) for several African countries as well as taxonomic notes on the enigmatic Madagascan endemic Mollugo caespitosa. New records are based on field investigations and/or the study of herbarium material. The structure of the article is based on the series of contributions dealing with new plant records for Asian and European countries and published in Acta Botanica Gallica/Botany Letters (Nobis et al. Citation2014a,b, Citation2015a,b, Citation2016). The taxa are presented alphabetically. Remarks on distribution, habitat, taxonomy, and delimitation are provided for each of the studied species, as well as a list of examined specimens. Geographical areas follow Brummit (Citation2001), herbaria codes follow Thiers (Citation2015).
Amaranthus muricatus (Gillies ex Moq.) Hieron. (Amaranthaceae)
Contributors – Duilio Iamonico, Ridha El Mokni
Distribution and habitat
Amaranthus muricatus is a South American species that is known as alien in other continents (see Boijan, Clements, and Borsch Citation2003; Mosyakin and Robertson Citation2003; Palmer Citation2009; Iamonico Citation2015a).
In Africa, this species was only known to occur in Morocco (APD Citation2016; Iamonico Citation2015a), but is here reported from Tunisia for the first time. According to Pyšek et al. (Citation2002) and Richardson and Pyšek (Citation2006), A. muricatus is considered as a casual alien species, growing on ruderal habitat such as roadsides, gardens and flowerbeds. The present note is part of an ongoing study of the Tunisian Amaranthaceae carried out by both contributors.
Taxonomic notes
Amaranthus muricatus belongs to the subgenus Albersia (Kunth) Gren. & Godr. sect. Pentamorion (Beck) Mosyakin & K.R.Robertson (Mosyakin and Robertson Citation1996), which differs from the related sections by having five perianth segments and indehiscent fruits. From the morphological point of view, A. muricatus is similar to Amaranthus induratus C.A. Gardner ex J. Palmer & Mowatt, the latter species being native to Australia (Palmer Citation2009). Both these taxa have (almost) linear leaves (an uncommon feature for Amaranthus), but A. muricatus has globose, clearly rugose and not ribbed fruits, whereas in A. induratus the fruits are ellipsoid, smooth or very slightly rugose and clearly longitudinally ribbed. Moreover, the perianth segments remain membranous at the fruiting stage in A. muricatus, but they harden and produce tooth-like projections in A. induratus (Palmer Citation2009; Iamonico Citation2015b).
Examined specimens (new records)
TUNISIA: Sousse (Bouficha, Centre of Tunisia), 36°17'33.08" N, 10°27'29.91" E, 6–9 m above sea level (a.s.l.), 18 August 2015, R. El Mokni (Herb. Univ. Bizerta); Sousse (Enfidha, Centre of Tunisia), 36°11'38.19" N, 10°26'00.45" E, 7–8 m a.s.l., 18 August 2015, R. El Mokni (Herb. Univ. Bizerta); Monastir (Jemmel, Centre of Tunisia), 35°37'05.88" N, 10°45'23.37" E, 18–31 m a.s.l., 18 August 2015, R. El Mokni (Herb. Univ. Bizerta); Monastir (Zeramdine, Centre of Tunisia), 35°33'54.52" N, 10°43'7.55" E, 90–95 m a.s.l., 18 August 2015, R. El Mokni (Herb. Univ. Bizerta).
Barleria oenotheroides Dum.Cours. (Acanthaceae)
Barleria flava J. Jacq., Barleria micans Nees, Barleria senegalensis Nees
Contributors – Santiago Martín-Bravo, Thomas F. Daniel
Distribution and habitat
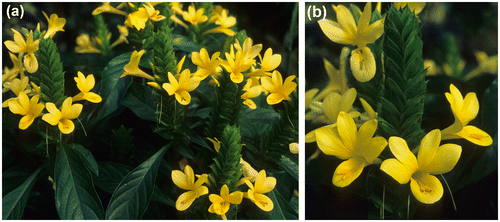
The giant yellow shrimp plant (Barleria oenotheroides; Figure ) displays a remarkable intercontinental, amphi-Atlantic disjunction between the Old and the New World tropics. It belongs to the large, predominantly tropical/subtropical African and Asian genus Barleria (c. 265 species: Balkwill and Balkwill Citation1998; Daniel et al. unpublished data), of which it is the only species present in the Neotropics. In addition, it constitutes one of only three presumed natural Old–New World disjunctions at the species level among the large family Acanthaceae (c. 4750 species: Daniel and McDade Citation2014). The native status of this species in America has been controversial and some authors consider it to have been introduced there (Hassler Citation2016). Recent molecular studies support the native status of B. oenotheroides in the New World and suggest a relatively ancient (probably Pliocene or Upper Miocene) long-distance dispersal from Africa to America, in spite of an unassisted dispersal syndrome, as the most plausible explanation for the origin of the disjunctive Neotropical populations (Martín-Bravo and Daniel Citation2016). The species grows in a variety of dry to wet forests. In Africa, it is frequently found along watercourses; in America, it also sometimes occupies weedy and disturbed habitats (e.g. roadsides, along trails). Barleria oenotheroides occurs in western tropical Africa and in the New World from Mexico to Venezuela (Daniel Citation1995; Balkwill and Balkwill Citation1998). Reference floristic works for Africa consistently agree on its presence in Guinea-Bissau, Sierra Leone, Ivory Coast and Ghana (Hutchinson et al. Citation2014; APD Citation2016; Hassler Citation2016). However, Hutchinson et al. (Citation2014) and Hassler (Citation2016) also report it for Mali and Nigeria, Hutchinson et al. (Citation2014) for Guinea and APD (Citation2016) for Liberia. Therefore, the African distribution of B. oenotheroides needed clarification. We have found herbarium specimens of this species from Senegal and Central African Republic, which implies new records for these countries. In addition, we have studied vouchers that confirm the presence of the species in Guinea, Liberia and Nigeria. On the other hand, we consider the records from Mali (see Hutchinson et al. Citation2014) as very doubtful. These collections were made by Chevalier in February 1905, allegedly in French Sudan (Chevalier Citation1920), which currently corresponds to Mali, but the actual collecting localities were in our opinion more probably located in current Guinea. Therefore, to our knowledge, the known African distribution of the species would be as follows: Senegal, Guinea-Bissau, Guinea, Sierra Leone, Liberia, Ivory Coast, Ghana, Nigeria and Central African Republic.
Taxonomic notes
The American populations were considered to be a different, Neotropical endemic species (Barleria micans Nees) from the west tropical African populations. Daniel (Citation1995) demonstrated the conspecificity of the disjunctive populations on the basis of morphology. Barleria oenotheroides is included in the subgenus Barleria and is the type species of sect. Chrysothrix M. Balkwill (c. 17 species; Balkwill and Balkwill Citation1997).
Examined specimens (including new records)
CENTRAL AFRICAN REPUBLIC: [Ouham-Pendé], route Bozoum [road to Bozoum], marigot [pond], February 1963, Bille 1104 (P!).
SENEGAL: [Ziguinchor], Bayottes (Casamance), 24 May 1957, J. Adam 13623 (P!); [Ziguinchor], Santiaba-Mandjak, J. Adam 13680 (MO!).
GUINEA: [Boké], bords du Batafon, Boké, G. Paroisse 16 (P!); [Faranah], Kouroussa, 14 June 1904, M. Pobéguin 921 (P!); [Kankan], chutes de la Sala [sala waterfall], 1956, J. Adam 11783 (P!); [Kindia], Friguiagbé, J. Adam 26750 (MO!); [Labé], Fouta Djallon, N de Labé [N of Labé], March 1950, R. Schnell 4751 (P!); [Mamou], Fololo, J. Adam 2873 (MO!); [Nzerekore], Macenta, Berge du Diani, J. Adam 3278 (MO!).
LIBERIA: [Grand Gedeh], along road from Tchien to Sinoe, c. 5 miles (8 km) south of Tchien, 18 January 1969, J. Jansen 1223 (MO!, NY!, P!).
NIGERIA: c. 6.5 miles (10.5 km) south of Okeigbo, and 1.5 miles (2.4 km) beyond Araromi, A. Jones & C. Onochie s.n. (FHI 14726 photo!).
Burkea africana Hook. (Fabaceae)
Contributor – Luís Catarino
Distribution and habitat
Burkea africana is a deciduous medium-sized tree 12–15(–20) m tall, widespread in tropical and southern Africa, mainly in the Sudano-Zambezian region from Senegal in the north, to Angola, Namibia, Botswana, Mozambique and northern South Africa in the south, and eastwards to Sudan and Uganda. It occurs in several types of savannahs and woodlands, mainly on well-drained, sandy and sandy loam soils, at 50–1750 m a.s.l. with an annual rainfall of 1000–1200 mm (Maroyi Citation2012).
This species is already known in west tropical Africa, for instance in Senegal and Guinea (Berhaut Citation1975; Lisowski Citation2009; Hutchinson et al. Citation2014) but is now recorded for the first time in Guinea-Bissau. It seems to be rare in the country and was found in woodland on a ferralsol, with c. 60% tree cover, dominated by Xeroderris stuhlmannii (Taub.) Mendonça & Sousa and Hexalobus monopetalus (A.Rich.) Engl. & Diels, but including also Bombax costatum Pellegr. & Vuillet, Cordyla pinnata (A.Rich.) Milne-Redh., Detarium microcarpum Guill. & Perr., Erythrophleum africanum (Benth.) Harms, Ficus exasperata Vahl, Lannea velutina A.Rich. and Parinari curatellifolia Planch. ex Benth. The annual rainfall at the collecting site is c. 1500 mm, and the altitude is c. 65 m a.s.l. Probably the species is here in its upper range of rainfall and lower range of altitude.
Taxonomic notes
Burkea africana is the single species in the genus.
Examined specimens (new record)
GUINEA-BISSAU: Dulombi National Park, near Paiai Lemenai, in woodland in a ferralsol, with c. 60% of tree cover, 11°51'5.7" N, 14°25'36.3" W, 65 m a.s.l., 11 December 2013, L. Catarino 2122 (LISC!).
Camptorrhiza strumosa (Baker) Oberm. (Colchicaceae)
Iphigenia strumosa Baker, Iphigeniopsis strumosa (Baker) Buxb., Iphigenia junodii Schinz, Iphigenia dinteri Schinz
Contributor – Alfred Maroyi
Distribution and habitat
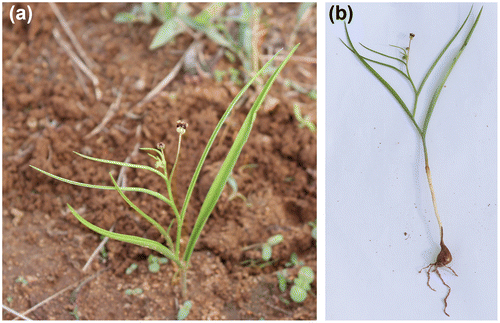
Camptorrhiza strumosa (Figure ) is a plant species confined to south tropical and southern Africa and characterized by relatively wide ecological amplitude. So far it has been recorded in open Brachystegia–Julbernadia woodland on sandy or light clay soils, and also in wet grasslands at medium to high altitude (up to 2000 m a.s.l.). In Zimbabwe, C. strumosa has hitherto only been recorded and mapped for the eastern, northern, southern and western floristic regions (Maroyi Citation2002; Hyde et al. Citation2015) that are distinct in terms of temperature, rainfall and altitude. In December 2014, the author undertook an expedition to the botanically poorly explored open woodlands of the central part of Zimbabwe. Plant material was collected and subsequently positively identified as C. strumosa. Despite a thorough survey of the area, only eight plants were found. These were growing close to each other in wooded grassland, in shallow loamy sandy soil dominated by Dichrostachys cinerea (L.) Wight & Arn., Terminalia sericea Burch. ex DC., Combretum imberbe Wawra and Acacia spp., at an altitude of 1375 m a.s.l. All the plants were small, 10 to 15 cm in height, and in bloom. These eight plants represent the new distribution and first record of C. strumosa for central Zimbabwe, significantly expanding the distribution range of the species in Zimbabwe. Therefore, C. strumosa occurs in all floristic regions of the country, and the taxon is more common than previously thought, although the discovered population consists of only a few individuals.
Taxonomic notes
The genus Camptorrhiza is a monotypic genus restricted to southern Africa where it is represented by C. strumosa in Angola, Botswana, Namibia, Mozambique, South Africa, Swaziland, Zambia and Zimbabwe (Maroyi Citation2002; Lekhak, Surveswaran, and Yadav Citation2015; Govaerts Citation2016; Govaerts and Persson Citation2016).
Examined specimens (new record)
ZIMBABWE: Central Zimbabwe, Shurugwi district, Ndaba village, in wooded grassland, 32°58'21" S, 27°55'06" E, 1375 m, 23 December 2014, A. Maroyi 1219 (UFH).
Caylusea abyssinica (Fresen.) Fisch. & C.A. Mey. (Resedaceae)
Reseda abyssinica Fresen.
Contributor – Santiago Martín-Bravo
Distribution and habitat
Caylusea abyssinica is a highland plant (c. 1200–2750 m a.s.l.), widely distributed in south, east and northeast tropical Africa, where it is present in a number of countries (Eritrea, Ethiopia, Kenya, Malawi, Mozambique, Rwanda, Sudan, Uganda, Tanzania; Robyns Citation1951; Elffers and Taylor Citation1958; Taylor Citation1958; Abdallah and de Wit Citation1978; de Wit Citation2000; da Silva, Izidine, and Amude Citation2004; APD Citation2016; Hassler Citation2016). It also marginally reaches the mountains of the southern Arabian Peninsula (Yemen), where it is very rare (Abedin Citation1986; Miller Citation1996). We are not aware of any reliable records of this species from Burundi and Zambia (Bingham et al. Citation2016) and records from Sudan (Abdallah and de Wit Citation1978; Darbyshire et al. Citation2014) should also be confirmed. It often becomes weedy in disturbed and ruderal sites, such as roadsides, open grassland or cultivated fields, although it has not been reported as an aggressive weed (Elffers and Taylor Citation1958; Abdallah and de Wit Citation1978). The cited specimen, which was collected in northeastern Zambia, is a new record for this country.
Taxonomic notes
Caylusea is a natural, well-defined genus on the basis of morphological and molecular characters (Abdallah and de Wit Citation1978; Martín-Bravo et al. Citation2007). It comprises three species: Caylusea abyssinica, Caylusea hexagyna (Forssk.) M.L.Green and Caylusea latifolia P.Taylor, the latter only known from its type locality (Elffers and Taylor Citation1958; Taylor Citation1958; de Wit Citation2000; Abdallah and de Wit Citation1978). These three species are distinguished mainly by the surface of the seed coat (papillose in Caylusea hexagyna and Caylusea latifolia; wrinkled-rugose in Caylusea abyssinica) and the number of lobes of the upper petal (2–3 in Caylusea latifolia; 4–5 in Caylusea abyssinica; (5–)7–9 in Caylusea hexagyna; see keys and detailed descriptions in Elffers and Taylor Citation1958; Taylor Citation1958; Abdallah and de Wit Citation1978; de Wit Citation2000).
Examined specimens (new record)
ZAMBIA: [Northern province] Mbala District, Chipundu Village near Saisi Valley, growing on an anthill, not common, 5000 ft [c. 1525 m], 20 July 1970, M. Sanane 1304 (WAG-110384!).
Caylusea hexagyna (Forssk.) M.L.Green (Resedaceae)
Caylusea canescens (L.) A. St. Hil., Reseda hexagyna Forssk., Reseda canescens L. (1767 non 1753)
Contributor – Santiago Martín-Bravo
Distribution and habitat
Caylusea hexagyna is the most widespread species of the genus. It is a desert plant widely distributed in northern Africa, the Arabian Peninsula and western Asia. In Africa it has been reported from Algeria, Cape Verde islands, Chad, Egypt, Eritrea, Libya, Mauritania, Morocco and Sudan (Abdallah and de Wit Citation1978; de Wit Citation2000; Martín-Bravo Citation2011; Brundu and Camarda Citation2013; Dobignard and Chatelain Citation2013; Darbyshire et al. Citation2014; APD Citation2016; Hassler Citation2016). It has not been reported for Tunisia and Somalia, and its presence in Ethiopia, based on a single specimen cited by Abdallah and de Wit (Citation1978), is doubtful (Hassler Citation2016). APD (Citation2016) apparently contains records of Caylusea hexagyna from northwest Mali and Niger, although the presence of the species is not explicitly indicated. We have found two specimens from Niger in herbarium of the French National Museum of Natural History (P), which confirm the presence of the species in this country.
Taxonomic notes
See Caylusea abyssinica.
Examined specimens (new records)
NIGER: Air, Agadez, February 1932, A. Chevalier 43447 (P-05365330!); Route Arlit-Agadez, oasis à 30 km d’Agadez [oasis 30 km from Agadez], adventice de cultures [weed in cultivated field], 8 January 1975, P. Lavie 845 (P-05366321!).
Datura wrightii Regel (Solanaceae)
Contributor – Filip Verloove
Distribution and habitat
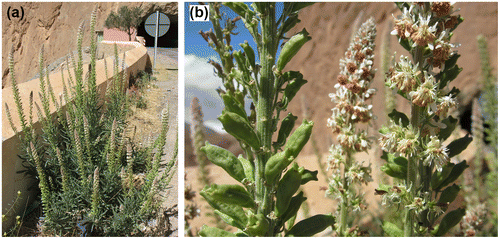
Datura wrightii (Figure ) is a species native to southwestern USA (Texas, California, Utah, Arizona, New Mexico and Colorado) and adjacent parts of northern Mexico. Its original distribution range, however, is unclear and even in parts of California and Utah (and doubtlessly elsewhere in the USA as well) it may have been introduced (Welsh et al. Citation1993; Baldwin et al. Citation2012). Although grown as an ornamental plant, D. wrightii does not seem to have become widely distributed throughout the world as a weed. It is well known from Australia where it has escaped from cultivation and naturalized locally (Haegi Citation1976). In Europe D. wrightii is probably a relatively recent introduction. It is known since 1979 from Corsica and is also present in continental France where it was long confused with Datura inoxia Mill. (Lambinon Citation2006). In Spain (Huesca province) it was first recognized in 2007 but it surely had been overlooked before (Verloove Citation2008). The same applies to Italy where it was first reported by Verloove et al. (Citation2010) (regions Lombardia and Emilia-Romagna) with the earliest records dating back to the 1960s (see also Montanari and Marconi (Citation2010) and Lazzaro et al. (Citation2013) for additional records). In Hungary it was reported by Király et al. (Citation2009) and it is also known from several regions in Austria (Walter et al. Citation2002). In recent years D. wrightii was also observed in Sardinia (Podda et al. Citation2012) and the Balearic Islands (Sáez et al. Citation2015). In most of these countries it is well-naturalized, and populations of D. inoxia classified as ‘invasive’ may well belong to this species (see below).
Figure 3. General habit of Datura wrightii. Photo taken in Morocco, Marrakesh–Tensift–Al Haouz, bare sandy area close to Issil river, June 2012, by F. Verloove.

In June 2012 a large population of D. wrightii was discovered on open rough ground on sandy substratum adjacent to River Issil in the suburbs of Marrakesh (Morocco), apparently for the first time in Africa. The species grew together with D. inoxia. As elsewhere in the Mediterranean it may have been overlooked so far in northern Africa. However, in some areas where D. inoxia is a commonly naturalized and invasive weed (e.g. in the Canary Islands), the absence of D. wrightii seems genuine (pers. obs.).
In its area of origin D. wrightii grows in sandy, loamy or gravelly open areas, up to an altitude of 2200 m (Baldwin et al. Citation2012), sometimes also in canyons (Welsh et al. Citation1993). In Australia it is found on vacant blocks in built-up areas, or on roadsides near homesteads in pastoral areas (Haegi Citation1976). In southern Europe it has been recorded in a wide range of disturbed habitats: gravelly river banks, roadsides, rough grounds, coastal pinewood, etc. It always grows in sun-exposed, open places, usually on sand or gravel, so in circumstances similar to those in its native distribution range.
Taxonomic notes
Datura wrightii has long been confused with D. inoxia. It is distinguished by the presence of numerous very short, eglandular hairs, the stigma that is well exserted past the anthers and the very large corollas 14–26 cm long. Datura inoxia, in contrast, has an indumentum that consists solely of long erect glandular hairs, the stigma is well below the anthers and the corollas are only 12–16 cm long.
Other names that have been misapplied to D. wrightii are Datura meteloides Dunal and Datura metel L. (Ewan Citation1944). The latter is a distinct species with capsules covered with blunt and short tubercles (instead of longer, sharp spines), while D. meteloides is a taxonomic synonym of D. inoxia (Barclay Citation1959).
Datura wrightii has the largest corollas among other Datura species and, of all the perennial species offered in horticulture, doubtlessly is the most attractive. It is likely to be more widespread than the other species, in gardens as well as in the wild (as an escape).
Examined specimens (new record)
MOROCCO: Marrakesh, Marrakesh–Tensift–Al Haouz, route Golf Royal, close to Issil river, rough ground, several tens, 14 June 2012, F. Verloove 9484 (BR!).
Digitaria ciliaris (Retz.) Koeler (Poaceae)
Contributor – Filip Verloove
Distribution and habitat
Digitaria ciliaris is a weedy species, found in open, disturbed areas in most warm temperate and tropical regions of the world. Although still often overlooked, it is now known as a naturalized alien in many parts of southern Europe (see Wilhalm [Citation2009] for an extensive overview). In some areas, for instance in the Canary Islands, it is much more frequent than the related and very similar Digitaria sanguinalis (L.) Scop. Surprisingly, it is hardly known in northwestern Africa where it may have been overlooked. In 2007 it was recorded for the first time in Algeria (Zeddam and Scholz Citation2007), but it was believed to be absent from Morocco and Tunisia (e.g. Dobignard and Chatelain Citation2010).
In 2012 Digitaria ciliaris was found as a plantation weed in the city of Agadir, Morocco, apparently for the first time in this country. However, a revision of caesura herbarium specimens of Digitaria sanguinalis from Morocco stored in the herbarium of the Botanic Garden of Meise, Belgium (BR), yielded an additional record of Digitaria ciliaris from El Menzeh where it was found in 1974 in similar circumstances. This species is probably widely neglected in Morocco and elsewhere in northwestern Africa.
Digitaria ciliaris grows in a wide range of disturbed habitats. It can be particularly abundant in plantation lots, arable fields and plant nurseries, especially those regularly irrigated.
Taxonomic notes
Digitaria ciliaris appears to be the (sub-)tropical counterpart of the more temperate Digitaria sanguinalis. Typical plants of both species are easily distinguished. Digitaria ciliaris has smooth marginal nerves of lower lemmas, without minute spines or with very few spinules in the upper one-third, a lower glume usually longer than 0.2 mm and upper side of leaves usually glabrous or with some long scattered hairs near base. Digitaria sanguinalis, in contrast, has marginal nerves of lower lemmas with minute spinules, more or less throughout, lower glume usually shorter than 0.2 mm and upper side of leaves usually hairy throughout (rarely glabrous). However, more or less intermediate forms sometimes occur and the specific boundaries may blur (probably as a result of introgression) since both species increasingly occur sympatrically. Gene flow between Digitaria species is a well-known phenomenon (Fontana et al. Citation2015).
Examined specimens (new records)
MOROCCO: Agadir, Boulevard Mohammed V, plantation weed, 22 June 2012, F. Verloove 9941 (BR!); Menzeh, pépinière, 28 June 1974, A. Vanderweyen 740628/1 (BR!; sub D. sanguinalis).
Mollugo caespitosa Scott Elliot (Molluginaceae)
Contributors – Alexander P. Sukhorukov, Maria Kushunina & Irina V. Belyaeva
Distribution and habitat
The genus Mollugo is distributed worldwide, predominantly in the tropics; however, many species have small distribution areas. Currently there are three Mollugo species in Madagascar (APD Citation2016): Mollugo nudicaulis Lam., and two endemics, Mollugo decandra Scott Elliot and Mollugo caespitosa Scott Elliot. Mollugo decandra is the only shrubby species in the genus and is usually found in open grasslands, whereas M. nudicaulis grows in disturbed habitats. Molluga caespitosa is known only from the locality mentioned in the protologue.
Taxonomic notes
Both M. decandra and M. caespitosa were accepted in their specific rank by Clifton (Citation2002), even though M. caespitosa is known from a single specimen (“Madagascar, dry, sandy arid country of Antandroi (Interior), June-July [without year], G.F. Scott Elliot 2978, K-000232028”). It consists of two small fragments with several obovate leaves and flower buds mounted on the same herbarium sheet. The characters indicated in the protologue (Scott Elliot Citation1893) are non-specific, could apply to any of the species of the genus Mollugo and do not provide sufficient information about the habit of M. caespitosa. It is believed that M. caespitosa is a perennial herb or small subshrub with a short dense inflorescence with several flowers (Scott Elliot Citation1893).
Scott Elliot (Citation1893) indicated that M. caespitosa is a relative of the annual M. nudicaulis, which is clearly heterogeneous in Madagascar, and its taxonomy needs to be clarified (Sukhorukov & Kushunina, in prep.). We assume that M. caespitosa is in fact an annual plant, similar to M. nudicaulis. However, the collected plants look like they have been overgrazed and so show an unusual habit similar to perennial herbs. Besides, the only known specimen of M. caespitosa does not include developed flowers or fruits, which are very important in the identification of Mollugo species. The image in the protologue (table 7 in Scott Elliot, l.c.) with the legend “Mollugo caespitosa” shows a shrub with the same morphological characters as M. decandra. The original material of M. caespitosa, composed of the herbarium collection and the plate, is definitely heterogeneous, representing two different taxa, one a deformed plant of M. nudicaulis without developed flowers or seeds and the other an adequate drawing of M. decandra. To avoid further confusion, we here lectotypify M. caespitosa with the illustration given by the author in the protologue and synonymize it with M. decandra.
Mollugo decandra Scott Elliot, J. Linn. Soc. London (Botany): 29(1): 24 (Citation1893).
Lectotype (Sukhorukov & Belyaeva, designated here): [Madagascar] Fort Dauphin [Tôlanaro], sand near sea, April 1890, G.F. Scott Elliot 2481 (K-000232027! isolecto – P!).
Protologue citation: “Sea-shore near Fort Dauphin, April, 2481!”
Comments: The protologue citation does not fulfil the requirements of Art. 9.1 of the ICN (McNeill et al. Citation2012). There is no sufficient indication that Scott Elliot used only this particular specimen when he described Mollugo decandra; there is the other specimen at P from the same locality collected by Scott Elliot with the label: “Fort Dauphin, sand hills, Scott Elliot” that also belongs to the original material. Following this, the specimen K-000232027 cannot be the holotype but rather one of the syntypes, which we designate here as the lectotype (Art. 9.2 and 9.11, McNeill et al. Citation2012).
=Mollugo caespitosa Scott Elliot, J. Linn. Soc. London (Botany): 29(1): 25 (Citation1893), syn. nov.
Lectotype (Sukhorukov & Belyaeva, designated here): [icon] “Mollugo caespitosa”, plate 7 in Scott Elliot (Citation1893), J. Linn. Soc. London (Botany) 29(1).
Ochradenus baccatus Delile (Resedaceae)
Contributor – Santiago Martín-Bravo
Distribution and habitat
Ochradenus is a genus composed of about nine species of desert shrubs distributed in northeast Africa, the Middle East and southwest Asia, although it is more diversified in the horn of Africa and especially in the southern part of the Arabian Peninsula (Abdallah and de Wit Citation1978; Miller Citation1984; Thulin Citation1994; Miller and Morris Citation2004; Martín-Bravo et al. Citation2007). Ochradenus baccatus is by far the most widely distributed species, as it spans almost all the genus distribution. It occupies desert and semi-desert habitats, frequently on rocky or stony grounds. In Africa it has been reported from Libya, Chad, Egypt, Sudan, Eritrea, Ethiopia and Somalia (Abdallah and de Wit Citation1978; Miller Citation1984; Thulin Citation1993; de Wit Citation2000; Martín-Bravo Citation2011; Brundu and Camarda Citation2013; Dobignard and Chatelain Citation2013; Darbyshire et al. Citation2014; APD Citation2016; Hassler Citation2016). Djibouti was listed by Hassler (Citation2016) and there seem to be records in APD (Citation2016), but the species is not explicitly indicated as present in the country. In addition, it was not recorded for Djibouti in relevant monographs or floras (Abdallah and de Wit Citation1978; Miller Citation1984; de Wit Citation2000). Therefore, we cite the specimen below as confirmation for the country.
Taxonomic notes
Ochradenus baccatus may be distinguished from other species in the genus by its baccate mature fruits, which are unique within the family, and are eaten and dispersed by birds, mammals and reptiles (endozoochory; Altstein Citation2005; Bronstein et al. Citation2007). The only other species of the genus present in Africa is Ochradenus randonioides Abdallah (= Ochradenus somalensis Baker f.), from which O. baccatus may be readily distinguished, aside from the fruit, by its minutely papillose seeds, longer leaves and by the green or yellowish young branches (Abdallah and de Wit Citation1978; Miller Citation1984; Thulin Citation1993; de Wit Citation2000).
Examined specimens (new records)
DJIBOUTI: Tadjourah, bas d’′escarpement sur basalte [base of escarpment on basalt], 8 April 1985, J. César 2194 (P-04724646!).
Reseda arabica Boiss. (Resedaceae)
Reseda praetervisa Müll.Arg.
Contributor – Santiago Martín-Bravo
Distribution and habitat
Reseda arabica is widely distributed in desert regions of North Africa and southwest Asia. It grows in arid steppes and wadis, on sandy or gravelly soils, from sea level to 1200 m a.s.l. (Martín-Bravo Citation2009). In Africa it has been reported to date from Morocco, Algeria, Tunisia, Libya and Egypt (Abdallah and de Wit Citation1978; Dobignard and Chatelain Citation2013; Martín-Bravo Citation2011; APD Citation2016; Hassler Citation2016). The presence of the species in Mauritania has not been indicated in any of these works, although APD (Citation2016) apparently contains a few records from northern Mauritania. During the taxonomic revision of Reseda sect. Phyteuma Lange we have found two specimens from Mauritania housed in the herbarium of the French National Museum of Natural History (P). They were collected in the Adrar region (Central Mauritania), not far from Atar, near the southeast border of western Sahara. This confirms the presence of the species in Mauritania and considerably expands the species range to the southwest.
Taxonomic notes
This species is included in section Phyteuma, which has 16 species and is the second largest in the genus after section Reseda. It is a monophyletic group based on molecular data (Martín-Bravo et al. Citation2007; Martín-Bravo and Jiménez-Mejías Citation2009). Its taxonomy is characterized by certain problems regarding species circumscription and the presence of hybridization processes (Martín-Bravo and Jiménez-Mejías Citation2009). In particular, R. arabica is probably one of the putative ancestors of the hybrid species R. odorata L., as inferred from the analysis of nuclear and plastid DNA sequences (Martín-Bravo et al. Citation2007; Martín-Bravo and Jiménez-Mejías Citation2009). Nonetheless, R. arabica is morphologically well-characterized and can be readily distinguished from closely related species by its persistent staminal filaments and the sessile or subsessile capsules (Martín-Bravo Citation2009). Reseda arabica, together with R. phyteuma L. (the latter distributed in the west and central Mediterranean region and central Europe), is the only widely distributed species in the section, which is mainly composed of restricted endemics from northwest Africa and southeast Mediterranean region (Martín-Bravo Citation2009).
Examined specimens (new records)
MAURITANIA: [Adrar region] El Wad, bord d’′oued [wadi border], 20°32'6" N, 12°14'12" W, sable [sand], 19 February 1996, Lamarche 911 (P-05388645!); Idem, W Ouelb el Raoui, 20°20'8.13" N, 12°46'6.94" W, bord de cunette, argile/sable [roadside, clay/sand], 6 February 1995, Lamarche, Lehlou & Sakho 21 (P-05388640!).
Reseda villosa Coss. (Resedaceae)
Reseda sudanica A.Chev.
Contributor – Santiago Martín-Bravo
Distribution and habitat
Reseda villosa (Figure ) is a Saharan endemic species that grows in arid and semi-arid deserts, on rocky or stony soils, frequently on slopes and roadsides. It is present in several North African countries but its distribution needs clarification. Reference chorological databases consistently agree on its presence only in Morocco, Mauritania, Algeria and Libya (Dobignard and Chatelain Citation2013; APD Citation2016; Hassler Citation2016). Our bibliographic survey indicates that Chad can be confidently added to this list (Abdallah and de Wit Citation1978; Brundu and Camarda Citation2013; Hassler Citation2016). The presence of R. villosa in Mali and Niger was indicated by Hutchinson et al. (Citation2014) and Hassler (Citation2016), respectively, but not by APD (Citation2016), although the latter apparently contains records from both countries. Burkill (Citation1985) gave a doubtful record for Senegal. The species has never been reported from Tunisia or Egypt and its presence in Sudan is based on a single specimen cited by Abdallah and de Wit (Citation1978). We have studied this specimen and the label says it was collected in “French Soudan” (near Goundan, small seasoned flood plain, 1 August 1959, G. Popov 56, BM-882908!), which probably confused Abdallah and de Wit, because this region currently corresponds to Mali. Furthermore, we have located the cited locality (Goundan) in Mali. Therefore, we propose the removal of Sudan from this species’ range. The specimens from the herbarium of the French National Museum of Natural History (P) cited below confirm its presence in Niger and Mali. Therefore, the current known distribution of R. villosa should be as follows: Morocco, Mauritania, Algeria, Mali, Niger, Libya, and Chad.
Taxonomic notes
Reseda villosa is included in the type section and is morphologically very distinctive. The closely related Moroccan endemic Reseda elata Coss. ex Müll.Arg. is the only species it can be confused with. However, R. villosa may be distinguished by the more or less densely pubescent stems and leaves and by the upper petal with a central lobe much longer and wider than the cockscomb-like small lateral lobes (Abdallah and de Wit Citation1978).
Examined specimens (new records)
NIGER (selected specimens): Takalam, 10 September 1987, Saadou 2322 (P-04724510!); Vallée de l’′Azar, sablo-limoneux [sandy-silty], 25 November 1969, G. Rippstein 80 (P-05365312!); Guest Tillia, bord depression de In Tarekenat, glaise limono-sableuse en bas de pente [border of In Tarekenat depression, sandy-silty clay at the bottom of a slope], 10 September 1985, Peyre de Fabrégues 4124 (P-05365313!); Egarek, bas fond sableux [sandy depression], 26 April 1968, Peyre de Fabrégues 2689 (P-05365315!, P-05365316!); In Todok, bas fond [depression], 17 January 1968, Peyre de Fabrégues 2665 (P-05365317!); P. Lavie 933 (P-05365318!); Air, Kori Tagueï, 13 February 1976, sable [sand], P. Lavie 982 (P-05365319!); Air, Kori Ofoud, 13 February 1976, sable [sand], P. Lavie 983 (P-05365320!); Air, Massif de l’′Ofoud, plaine de altitude [plateau], 1300 m, 14 February 1976, sable [sand], P. Lavie 986 (P-05365322!); entre Tabello et Agades [between Tabello and Agadez], March 1933, Petit-Lagrange 55 (P-05365323!).
MALI: Adrar des Iforas, 1983, E. ag Sidiyène 142 (P-00580913!); Region de Tombouctou, Gassa, 21 August 1899, A. Chevalier 2521 (P-00086077!, B-100165036!); Azigui, Oued d'Asskarai, 1934, Mission d'Études de la Biologie des Acridiens 500 (P-00580914!, P-005809151!, P-005809161!).
Conflict of interest
No conflict of interest was declared by the authors.
Notes on contributors
Alexander P. Sukhorukov, Dr Sci since 2016, has been the leading Scientist at the Department of Higher Plants, Biological Faculty (Moscow Lomonosov State University), Professor of Chinese Academy of Sciences (since 2012), Member of the Russian Botanical Society and Botanical Society of America. Sukhorukov’s main research fields are flora of Russia; flora of Himalaya; flora of Africa; anatomy; taxonomic, carpological and molecular revisions of Chenopodiaceae, Molluginaceae, Nyctaginaceae (all – Caryophyllales), and Asteraceae. Sukhorukov is the author or co-author of the taxonomic treatments of some families in the manual identifications of European Russia and Caucasus, as well as Flora of China, Flora of Iraq, Flora of Palestine, Flora of Russia (general editor), Flora of Central Africa projects. Contribution: the idea and project coordination, revision of herbarium collections, preparation of the manuscript.
Santiago Martín-Bravo has a PhD in Botany (2009). Bravo is a researcher and assistant professor at the Botany Area of the Department of Molecular Biology and Biochemical Engineering at Universidad Pablo de Olavide (Seville, Spain). Bravo is a curator of UPOS herbarium. Bravo’s main research interests are on systematics and evolution of angiosperms, using multidisciplinar approaches ranging from classic taxonomy to molecular phylogenetics, estimation of divergence times, cytogenetic evolution, phylo- and biogeographic analyses. Bravo is a taxonomic expertise in Resedaceae and Carex (Cyperaceae). Contribution: study of herbarium specimens, writing of the manuscript.
Filip Verloove is a researcher at Botanic Garden of Meise, Belgium. Verloove’s main research fields are non-native vascular plants in western and southern Europe (mainly Belgium, France, Italy, Portugal and Spain + Macaronesia), with emphasis on complex or poorly understood taxa (often Cyperaceae and Poaceae). Verloove is the author of the Flora of Belgium and of numerous scientific publications in national and international journals. Contribution: field research and/or revision of herbarium collections, preparation of selected parts of the manuscript.
Alfred Maroyi, PhD, is an associate professor at the Department of Botany, University of Fort Hare, South Africa. Maroyi’s main research fields are taxonomy and ecology of the Flora of Africa. Contribution: Field study, conducted research, analysed the results and wrote the manuscript.
Duilio Iamonico, graduated from the University of Rome Sapienza in Natural Science (since 2003) and Environmental Science (since 2006), post graduated master at the University RomaTRE in GIS and remote sinsing for geo-environmental design (since 2007), PhD in Environmental Design at the University of Rome Sapienza (since 2016). Curator of the Herbarium Flaminio (HFLA, http://sweetgum.nybg.org/ih/person.php?irn=175325), licensed Professor in Systematic Botany since 2013, supervisor of 37 Master thesis, including researches in Natural Sciences, and Environmental Planning. Iamonico is a member of Italian Botanical Society, group “Floristic, Systematic and Evolution”. Iamonico’s main research fields include: flora of Italy and Europe, nomenclature and taxonomy of many genera belonging to Amaranthaceae, Araceae, Asteraceae, Caryophllaceae, Chenopodiaceae, Malvaceae, Poaceae, Plantaginaceae, Plumbaginaceae, Rosaceae. Iamonico is the author of about 270 scientific papers plus about 50 participations as author at National and International Conferences. Iamonico is the coordinator or collaborator to national and international projects, Subject Editor of the journal Phytotaxa for the order Caryophyllales, referee for 14 scientific journals.
Luís Catarino, PhD, is a researcher at the Plant Biology Department, Faculty of Sciences, Lisbon University (since 2015), and member of the Centre for Ecology, Evolution and Environmental Changes (cE3c). Catarino’s main research interests include: tropical vegetation ecology, flora and land cover changes; ethnobotany, ethnoecology and sustainable use of natural resources; and protected areas management and monitoring. Catarino’s working subjects include vegetation ecology, flora, and natural resources assessment in Tropical Africa (particularly in Guinea-Bissau and Angola), and Tropical South America, carbon stocks assessment, vegetation mapping, inventory of medicinal flora and traditional medicine practices, databasing herbarium collections, and monitoring systems for protected areas. Contribution: field research and/or revision of herbarium collections, preparation of selected parts of the manuscript.
Ridha El Mokni is a teacher-researcher in Botany and Cryptogamy at the Faculty of Pharmacy of Monastir, Monastir University (since 2015). Mokni is a teacher-researcher in Botany, Lichenology, Mycology, Phytosociology and Forest Ecology at the Silvo-Pastoral Institute of Tabarka, Jendouba University (since 2012). Mokni is also a teacher-researcher in Botany and Plant Applied Ecology at the Faculty of Sciences of Bizerte, Carthage University (since 2001). Mokni is a member of the Tunisian Association Taxonomy (ATUTAX) since 2010. Mokni is also the author and co-author of several taxonomic research on some North African endemic taxa. Contribution: field research and/or revision of herbarium collections, preparation of selected parts of the manuscript.
Thomas F. Daniel, PhD, is Curator of Botany at the California Academy of Sciences (since 1986; currently Curator Emeritus), Research Professor of Biology at San Francisco State University (since 1998), Member of American Society of Plant Taxonomists and Botanical Society of America. Daniel’s main research interests include: systematic botany, Acanthaceae, floristics in Arizona and California, and cytology. Contribution: field and herbarium studies on Barleria oenotheroides.
Irina V. Belyaeva, PhD, FLS, is a content editor – Plant & Fungal Names, Biodiversity Informatics & Spatial Analysis, Science Directorate, Herbarium, Royal Botanic Gardens, Kew. Belyaeva’s research interest includes: Nomenclature & taxonomy, plant systematics & phylogeny (Salicaceae). Contribution: editing nomenclatural content.
Maria Kushunina is a researcher at Department of Plant Physiology, Biological Faculty, Lomonosov Moscow State University. Her main research interests are plant anatomy, plant distribution, plant mineral nutrition, plant taxonomy. Contribution: revision of herbarium collections, and preparation of the manuscript.
Funding
The research of Alexander Sukhorukov and Maria Kushunina was supported by the Russian Science Foundation (grant # 14-50-00029). Alfred Maroyi would like to express his gratitude to the National Research Foundation (NRF) of South Africa for financial support to conduct his research (grant T398).
Acknowledgements
Santiago Martín-Bravo thanks Maha Kordofani (University of Khartoum, Sudan) for comments on the distribution of Resedaceae in Sudan. The authors thank Keith Chamberlain (Rothamsted Research, UK) for linguistic corrections of the text.
References
- Abdallah, M. S., and H. C. D. de Wit. 1978. “The Resedaceae: a taxonomical revision of the family (final instalment).” Mededelingen Landbouwhogeschool Wageningen 78: 1–416.
- Abedin, S. 1986. “Contribution to the flora of Saudi Arabia III. A new species of Caylusea (Resedaceae).” Willdenowia 14: 433–436.
- APD (African Plants Database; version 3.4.0 2016). http://www.ville-ge.ch/musinfo/bd/cjb/africa/ (accessed March 29, 2016).
- Altstein, O. 2005. “Seed dispersal of a fleshy-fruited desert shrubby birds: effects of gut passage, disperser movement and local spatial structure on seed dispersion patterns.” MSc thesis, Ben-Gurion University of the Negev.
- Baldwin, B. G., D. H. Goldman, D. J. Keil, R. Patterson, T. J. Rosatti, and D. H. Wilker. 2012. The Jepson Manual: Vascular Plants of California. 2nd ed. Berkeley: University of California Press.
- Balkwill, M. J., and K. Balkwill. 1997. “Delimitation and infra-generic classification of Barleria (Acanthaceae).” Kew Bulletin 52: 535–573.
- Balkwill, M. J., and K. Balkwill. 1998. “A preliminary analysis of distribution patterns in a large, pantropical genus, Barleria L. (Acanthaceae).” Journal of Biogeography 25: 95–110.
- Barclay, A. S. 1959. “New considerations in an old genus: Datura.” Botanical Museum leaflets, Harvard University 18: 245–272.
- Berhaut, J. 1975. Flore illustrée du Sénégal, Tome IV. Dakar: Gouvernement du Sénégal.
- Bingham, M. G., A. Willemen, B. T. Wursten, P. Ballings, and M. A. Hyde. 2016. Flora of Zambia. http://www.zambiaflora.com/index.php (accessed March 29, 2016).
- Bojian, B., S. E. Clemants, and T. Borsch. 2003. “Amaranthus L.” In Flora of China, 5, edited by Z. Y. Wu, P. H. Raven and D. Y. Hong, 415–429. Beijing: Science Press.
- Bronstein, J. L., I. Izhaki, R. Nathan, J. J. Tewksbury, O. Spiegel, A. Lotan, and O. Altstein. 2007. “Fleshy-fruited plants and frugivores in desert ecosystems.” In Seed Dispersal: Theory and its Application in a Changing World, edited by A. J. Dennis, E. W. Schupp, R. J. Green and D. W. Westcott, 148–177. Wallingford: CABI Publishing.
- Brummit, R. K. 2001. World Geographical Scheme for Recording Plant Distribution. 2nd ed. Pittsburgh, PA: Hunt Institute for Botanical Documentation, Carnegie Mellon University.
- Brundu, G., and I. Camarda. 2013. “The Flora of Chad: a checklist and brief analysis.” PhytoKeys 23: 1–17.
- Burkill, H. M. 1985. The useful plants of west tropical Africa. vol. 4. London: Royal Botanic Gardens, Kew.
- Chevalier, M. A. 1920. Exploration Botanique de l’Afrique Occidentale Française. vol. 1. Paris: Paul Lechevallier.
- Clifton, R. 2002. Madagascar’s Plants: Checklist of the Endemic Flowering Plants. R. Clifton: Dover.
- da Silva, M. C., S. Izidine, and A. B. Amude. 2004. “A preliminary checklist of the vascular plants of Mozambique.” Southern African Botanical Diversity Network Report 30: 1–183.
- Daniel, T. F. 1995. “New and reconsidered Mexican Acanthaceae. IV. Chiapas.” Proceedings of the California Academy of Sciences 48: 253–284.
- Daniel, T. F., and L. A. McDade. 2014. “Nelsonioideae (Lamiales: Acanthaceae): revision of genera and catalog of species.” Aliso 32: 14–44.
- Darbyshire, I., M. Kordofani, I. Farag, R. Candiga, and H. Pickering. 2014. The Plants of Sudan and South Sudan. An Annotated Checklist. London: Royal Botanic Gardens, Kew.
- de Wit, H. C. D. 2000. “Resedaceae.” In Flora of Ethiopia & Eritrea, 2(1), edited by S. Edwards, M. Tadesse, S. Demissew and I. Hedberg, 170–176. Addis Ababa and Uppsala: Addis Ababa University and Uppsala University.
- Dobignard, A. and C. Chatelain. 2010. Index synonymique de la Flore d’Afrique du nord, Volume 1: Pteridophyta, Gymnospermae, Monocotyledonae. Genève: éditions des Conservatoire et Jardin botaniques de la Ville de Genève.
- Dobignard, A. and C. Chatelain. 2013. Index synonymique de la Flore d’Afrique du nord Volume 5: Dicotyledonae: Oleaceae-Zygophyllaceae. Genève: Éditions des Conservatoire et Jardin botaniques de la Ville de Genève.
- Elffers, J., and P. Taylor. 1958. “Flora of Tropical East Africa: Resedaceae.” edited by C. E. Hubbard and E. Milne-Redhead. London: Crown Agents for Oversea Governments and Administrations.
- Ewan, J. 1944. “Taxonomic history of perennial south-western Datura meteloides.” Rhodora 46: 317–323.
- Fontana, L. C., D. Agostinetto, A. C. Langaro, L. W. Pacheco Arge, J. J. Franco, and V. J. Bianchi. 2015. “Genetic diversity among crabgrass weed ecotypes (Digitaria spp.) occurring in field crops in Rio Grande do Sul, Brazil.” Australian Journal of Crop Science 9 (10): 931–939.
- Govaerts, R. 2016. “Camptorrhiza.” In Species 2000 & ITIS Catalogue of Life, edited by Y. Roskov et al. http://www.catalogueoflife.org/col (accessed March 29, 2016).
- Govaerts, R., and K. Persson. 2016. World Checklist of Colchicaceae. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet; http://apps.kew.org/wcsp/ (accessed March 29, 2016).
- Haegi, L. 1976. “Taxonomic account of Datura L. in Australia with a note on Brugmansia Pers.” Australian Journal of Botany 24: 415–435.
- Hassler, M. 2016. “World plants: Synonymic checklists of the vascular plants of the world (version Nov 2015)”. In Species 2000 & ITIS Catalogue of Life, edited by Y. Roskov et al. http://www.catalogueoflife.org/col (accessed March 29, 2016).
- Hutchinson, J., J. M. Dalziel, R. W. Keay, and F. N. Hepper. (1972) 2014. Flora of West Tropical Africa. eBook reprint of the 2nd ed.
- Hyde, M. A., B. T. Wursten, P. Ballings, and C. M. Palgrave. 2015. Flora of Zimbabwe: Species information. http://www.zimbabweflora.co.zw/speciesdata/species.php?species_id=113140 (accessed October 20, 2015).
- Iamonico, D. 2015a. “Amaranthaceae Juss.” In Euro+Med Plantbase – the information resource for Euro-Mediterranean plant diversity (accessed December 18, 2015). http://ww2.bgbm.org/EuroPlusMed/PTaxonDetail.asp?NameCache=Amaranthus&PTRefFk=7300000.
- Iamonico, D. 2015b. “Taxonomic revision of the genus Amaranthus (Amaranthaceae) in Italy.” Phytotaxa 199 (1): 1–84.
- Király, G., A. Baranyainagy, S. Kerekes, A. Király, and M. Korda. 2009. ““Additions to the non-indigenous flora of Hungary”. (in Hungarian).” Flora Pannonica 7: 3–31.
- Lambinon, J. 2006. “Datura wrightii.” In Notes et contributions à la flore de Corse, XXI, edited by D. Jeanmonod, and A. Schlüssel. Candollea 61: 130.
- Lazzaro, L., G. Ferretti, G. Galasso, L. Lastrucci, and B. Foggi. 2013. “Contributo alla conoscenza della flora esotica dell’Arcipelago Toscano, Italia [Contribution to the knowledge of the exotic flora of Tuscany Archipelago, Italy].” Atti della Società italiana di scienze naturali e del museo civico di storia naturale di Milano 154(I): 3–24.
- Lekhak, M. M., S. Surveswaran, and S. R. Yadav. 2015. “Generic identity of Camptorrhiza indica (Colchicaceae) based on cytogenetics and molecular phylogenetics.” Journal of Systematics and Evolution 54: 75–82.
- Lisowski, S. 2009. “Flore (Angiospermes) de la République de Guinée. Première partie (texte).” Scripta Botanica Belgica 41: 1–517.
- Maroyi, A. 2002. “Family Colchicaceae in Zimbabwe.” Kirkia 17: 1–10.
- Maroyi, A. 2012. “Burkea africana Hook.” In Plant Resources of Tropical Africa 7: timbers 2, edited by R. H. M. J. Lemmens, D. Louppe and A. A. Oteng-Amoako, 174–177. PROTA Foundation: Wageningen.
- Martín-Bravo, S. 2009. “Sistemática, evolución y biogeografía de la familia Resedaceae [Systematic, evolution and biogeography of the Resedaceae family].” Ph.D dissertation, Universidad Pablo de Olavide, Sevilla.
- Martín-Bravo, S. 2011. “Resedaceae.” In Euro+Med Plantbase – the information resource for Euro-Mediterranean plant diversity (accessed March 29, 2016). http://ww2.bgbm.org/EuroPlusMed/query.asp.
- Martín-Bravo, S., and T. F. Daniel. 2016. “Molecular evidence supports ancient long-distance dispersal for the amphi-Atlantic disjunction in the shrimp plants (Barleria, Acanthaceae).” American Journal of Botany 103 (6): 1103–1116.
- Martín-Bravo, S., and P. Jiménez-Mejías. 2009. “Molecular data helps traditional taxonomy: re-evaluation of Reseda collina (Resedaceae), and a new record for Europe.” Folia Geobotanica 44 (4): 399–421.
- Martín-Bravo, S., H. Meimberg, M. Luceño, W. Märkl, V. Valcárcel, C. Braüchler, P. Vargas, and G. Heubl. 2007. “Molecular systematics and biogeography of Resedaceae based on ITS and trnL-F sequences.” Molecular Phylogenetics and Evolution 44: 1105–1120.
- McNeill, J., F. R. Barrie, W. R. Buck, V. Demoulin, W. Greuter, D. L. Hawksworth, P. S. Herendeen, et al. 2012. “International code of nomenclature for algae, fungi and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011.” Regnum Vegetabile 154: 1–274.
- Miller, A. G. 1984. “A revision of Ochradenus.” Notes from the Royal Botanic Garden Edinburgh 41: 491–504.
- Miller, A. G. 1996. “Resedaceae.” In Flora of the Arabian Peninsula and Socotra, 1, edited by A. G. Miller and T. A. Cope, 448–460. Edinburgh: Edinburgh University Press.
- Miller, A. G., and M. Morris. 2004. Ethnoflora of the Socotra Archipelago. Edinburgh: Royal Botanic Garden.
- Montanari, S., and G. Marconi. 2010. “Segnalazioni floristiche in Romagna [Floristic reports in Romagna].” Quaderno di studi e notizie di storia naturale della Romagna 31: 1–10.
- Mosyakin, S. L., and K. R. Robertson. 1996. “New infrageneric taxa and combinations in Amaranthus (Amaranthaceae).” Annales Botanici Fennici 33: 275–281.
- Mosyakin, S. L., and K. R. Robertson. 2003. “Amaranthus L.” In Flora of North America North of Mexico (Magnoliophyta: Caryophyllidae, part 1), 4, 410–435. Oxford: Oxford University Press.
- Nobis, M., A. L. Ebel, A. Nowak, B. Paszko, A. A. Bobrov, Y. A. Kotukhov, A. N. Kupriyanov, et al. 2015b. “Contribution to the flora of Asian and European countries: new national and regional vascular plant records, 4.” Acta Botanica Gallica: Botany Letters 162 (4): 301–316.
- Nobis, M., A. L. Ebel, A. Nowak, O. T. Turginov, A. N. Kupriyanov, A. Nobis, et al. 2014b. “Contribution to the flora of Asian and European countries: new national and regional vascular plant records, 2.” Acta Botanica Gallica: Botany Letters 161 (2): 209–221.
- Nobis, M., A. Nowak, A. Nobis, B. Paszko, R. Piwowarczyk, S. Nowak, and V. Plasek. 2014a. “Contribution to the flora of Asian and European countries: new national and regional vascular plant records.” Acta Botanica Gallica 161 (1): 81–89.
- Nobis, M., A. Nowak, A. L. Ebel, A. Nobis, S. Nowak, P. D. Gudkova, et al. 2015a. “Contribution to the flora of Asian and European countries: new national and regional vascular plant records, 3.” Acta Botanica Gallica: Botany Letters 162 (2): 103–115.
- Nobis, M., A. Nowak, R. Piwowarczyk, A. L. Ebel, G. Király, M. Kushunina, A. P. Sukhorukov, et al. 2016. “Contribution to the flora of asian and european countries: new national and regional vascular plant records, 5.” Botany Letters 163 (2): 159–174.
- Palmer, J. 2009. “A conspectus of the genus Amaranthus L. (Amaranthaceae) in Australia.” Nuytsia 19: 107–128.
- Podda, L., V. Lazzeri, F. Mascia, O. Mayoral, and G. Acchetta. 2012. “The checklist of the Sardinian alien flora: an update.” Notulae Botanicae Horti Agrobotanici Cluj-Napoca 40 (2): 14–21.
- Pyšek, P., D. M. Richardson, M. Rejemánek, G. L. Webster, M. Williamson, and J. Kischner. 2002. “Alien plants in checklist and floras: towards better communication between taxonomist and ecologist.” Taxon 53 (1): 131–143.
- Richardson, D. M., and P. Pyšek. 2006. “Plant invasion: merging the concepts of species invasiveness and community invasibility.” Progress in Physical Geography 30: 409–431.
- Robyns, W. 1951. “Resedaceae.” In Flore du Congo Belge et du Ruanda-Urundi, 2, edited by le Comité exécutif de la flore du Congo Belge et le Jardin botanique de l'Etat, 544–546. Brussels: Publications de l'Institut national pour l'étude agronomique du Congo Belge.
- Sáez, L., G. Bibiloni, J. Rita, L. Gil, E. Moragues, C. Romero Zarco, and J. Vicens. 2015. “Additions and amendments to the flora of the Balearic Islands.” Orsis 29: 173–192.
- Scott Elliot, G. F. 1893. “New and little-known Madagascar plants, collected and enumerated by G.F. Scott Elliot.” Journal of the Linnean Society of London (Botany) 29 (1): 1–67.
- Taylor, P. 1958. “The genus Caylusea St.Hil. in Tropical Africa.” Kew Bulletin 13 (2): 283–286.
- Thiers, B. 2015. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/ (accessed March 15, 2016)
- Thulin, M. 1993. “Resedaceae.” In Flora of Somalia, 1, edited by M. Thulin, 76–81. London: Royal Botanic Gardens, Kew.
- Thulin, M. 1994. “A new species of Ochradenus (Resedaceae) from southern Arabia.” Nordic Journal of Botany 14: 383–384.
- Verloove, F. 2008. “Datura wrightii (Solanaceae), a neglected xenophyte, new to Spain.” Bouteloua 4: 37–40.
- Verloove, F., G. Galasso, E. Banfi, and N. M. G. Ardenghi. 2010. “34. Datura wrightii.” In Notulae alla flora esotica d’Italia [Notulae to the exotic flora of Italy], edited by C. Nepi, S. Peccenini, and L. Peruzzi. Informatore Botanico Italiano 42(1): 388.
- Walter, J., F. Essl, H. Niklfeld, and M. A. Fischer. 2002. “Gefäßpflanzen [Vascular Plants].” In Neobiota in Österreich [Neobotia in Austria], edited by F. Essl and W. Rabitsch, 46–173. Wien: Umweltbundesamt.
- Welsh, S. L., N. D. Atwood, S. Goodrich, and L. C. Higgins, eds. 1993. A Utah Flora. Provo, UT: Brigham Young University.
- Wilhalm, T. 2009. “Digitaria ciliaris in Europe.” Willdenowia 39: 247–259.
- Zeddam, A., and H. Scholz. 2007. “Digitaria ciliaris.” In Med-Checklist Notulae, 26, edited by W. Greuter, and T. Raus. Willdenowia 37: 441.