Abstract
Potential habitat characterizations for past (Late Glacial Maximum), present and future scenarios were calculated for Iberian species of D. pungens gr. (D. pungens subsp. pungens, D. pungens subsp. langeanus, D. pungens subsp. gredensis, D. pungens subsp. brachyanthus and D. pungens subsp. hispanicus). Results were discussed according to Allison’s interpretation of insurance theory, where diversity is contrasted with resilient behaviours. Resilience is here defined as the diversification of responses to assure that functionality. The discussion of results was also compared with the morphological characterization. Environmental results confirmed the morphological similarity between these taxa. An apparent geographic divergence was observed: D. pungens subsp. hispanicus and D. pungens subsp. pungens supported an eastern response, while D. pungens subsp. langeanus and D. pungens subsp. gredensis supported a western response. The intermediate response for those extremes was supported by D. pungens subsp. brachyanthus. Based on these results, resilience in this genetic pool is concentrated on D. pungens subsp. gredensis and D. pungens subsp. hispanicus, while D. pungens subsp. langeanus, D. pungens subsp. brachyanthus and D. pungens subsp. pungens showed their resilience strategies to guarantee responses along the eastern, northern and western mountain systems for current environmental conditions only.
Introduction
Crespí et al. (Citation2007) characterized the morphology of the Iberian taxa included in Dianthus pungens L. (D. pungens subsp. pungens – identified from this point on as Dianthus pungens; D. pungens subsp. langeanus – identified from this point on as D. langeanus; D. pungens subsp. gredensis – identified from this point on as D. gredensis; D. pungens subsp. brachyanthus – identified from this point on as D. brachyanthus; and D. pungens subsp. hispanicus – identified from this point on as D. hispanicus). This is a common mountain and alpine group of species – with the exception of D. hispanicus, which occurs up to the Mediterranean coast (Bernal, Laínz, and Garmendia Citation1990). The diverse set of taxonomical and morphological traits found in these taxa is useful in revealing the apparent evolutionary patterns that these subspecies have suffered in the Iberian Peninsula, although it is difficult to distinguish the individuals of this group. Two clear morphological strategies have been observed (Crespí et al. Citation2007): (a) a morphological overlap between D. langeanus, D. gredensis and D. brachyanthus; and (b) smaller similarities between D. brachyanthus and D. hispanicus.
It has to been observed that these subspecies are present in northern Morocco (D. brachyanthus), and that the representative species of this group, D. pungens, is mainly distributed outside of the Iberian Peninsula (South France), occurring sparsely in the oriental Pyrenees (along the border of Spain and France). This biogeographic pattern may contribute to a better understanding of the holo-pleistocenic floristic dynamics in the Iberian Peninsula.
This corroborates the Glacial North–South and Inter-glacial South–North dynamics in previous studies (Bhagwat and Willis Citation2008; Linares Citation2011). Nevertheless, there is an inherent biogeographic complexity associated with these floristic dynamics, which involve genetic divergences along the Iberian Peninsula (Carvalho et al. Citation2016; Gómez and Lunt Citation2007; Hampe et al. Citation2003; Olalde et al. Citation2002; Picó et al. Citation2008; Rodríguez-Sánchez et al. Citation2010). This behaviour clearly distinguishes the peninsula between West and East, which this study compared with potential distribution models under past climate scenarios (18.000–12.000bc) and future climate change scenarios (2070). The results were interpreted using the resistance and resilience concepts of insurance theory (Allison Citation2004; Rocha et al. Citation2016). Resistance is here defined as the ability to maintain functionality under environmental change, while resilience is defined as diversity of responses to those changes in order to restore functionality (Brand Citation2009; Stringham, Krueger, and Shaver Citation2001). According to this definition, resistance is here understood as the ability of each species to persist under climate change, while resilience is understood as the diversity of responses in the Iberian group of D. pungens. In the present work, the response to predicted future change scenarios is described as the resilience capacity of every taxon analysed to face those environmental variations. We analysed the variation of areas that could potentially shelter these taxa, as well as the overlap between areas of distribution.
Methods
In this study, we used the same plant materials as those used by Crespí et al. (Citation2007). A total of 461 individuals, stored in 13 herbaria (AVE, BC, BCF, BRESA, COI, FCO, HVR, JACA, LEB, LISE, LISU, MA, MAF, PO, SALA, SANT and SEV; herbaria designations and addresses are in accordance with Index Herbariorum codes, Thiers 2010). The distribution of individuals per taxon was as follows: 198 for D. brachyanthus; 90 for D. langeanus; 20 for D. gredensis; 6 for D. pungens; and 153 for D. hispanicus.
The study area comprised the Iberian Peninsula, with elevation ranging from 50 m (D. hispanicus, MA367957, Ab: Jávea, playa de la Granadilla) to 3400 m (D. brachyanthus, BCF42828, Gr: Sierra Nevada, Faldas del Veleta).
Environmental data matrices were compiled relating points with collected specimens to variables from the WORLDCLIM v.1.4 data set (at http://www.worldclim.org; Hijmans et al. Citation2005) for past (approximately 22,000 years BC), current and future (2070) conditions. Temperature (monthly values), precipitation (average values) and elevation were the selected variables.
WORLDCLIM past and future conditions are derived from general circulation models, specifically MIROC-ESM for the Last Glacial Maximum (LGM, approximately 22,000 years ago) and MIROC5 for the year 2070, under several Representative Concentration Pathways (RCPs). These GCM climate projections in WORLDCLIM are the most recent and are used in the Intergovernmental Panel on Climate Change’s (IPCC’s) Fifth Assessment Report – AR5 (IPCC AR5 WG1 Citation2013). RCPs are four greenhouse gas concentration trajectories adopted by the IPCC for AR5 in 2014 (Moss et al. Citation2008), which describe different climate scenarios depending on the quantity of greenhouse gas emissions. The four RCPs – RCP 2.6, RCP 4.5, RCP 6, and RCP 8.5 – are named after a possible range of radiative forcing values in the year 2100 relative to pre-industrial values (+2.6,+4.5,+6.0, and +8.5 W/m2, respectively) (Weyant et al. Citation2009).
In this study, we selected two IPCC RCPs (RCP 4.5 and RCP 8.5). In the RCP 4.5 scenario, emissions peak at around 2040, and then decline, while in the RCP 8.5 scenario, emissions continue to rise throughout the twenty-first century (Meinshausen et al. Citation2011). RCP 4.5 2070 represents a gentler scenario compared to RCP 8.5 2070.
Comparative statistical analysis was carried for morphological variables (based on the same protocol as that applied by Crespí et al. [Citation2007]) and for environmental conditions d of each scenario. The morphological results were shown by Unweighted Pair-Group Average (UPGA)-City block clusters and a “forward stepwise” Discriminant Canonical Analysis (DCA), as in Crespí et al. (Citation2007).
In order to select an adequate environmental matrix for analysis, we determined the influence of each variable under each scenario. The environmental matrix for that characterization is the combination of those variables. DCA was carried out on the data matrix, from which it is possible to discriminate the influence of the selected variables to describe the environmental change of the different environmental scenarios (past, current and future).
Results
The morphological comparison between taxa is shown in Figure a (cluster analysis) and in Figure b (DCA). In order to relate these results with environmental analysis, a phenogramatic line is drawn for the first three groups (with sufficient number for statistical comparisons). Three groups are hence described: (i) D. langeanus-D. gredensis; (ii) D. hispanicus; and (iii) D. brachyanthus–D. pungens. The most discriminant morphological variables, obtained by the DCA, are shown in Table .
Figure 1. (a) Dendrogram representation of the cluster analysis for the average morphological behaviours for every taxa analysed; and (b) DCA representation for the first roots per taxa analysed.
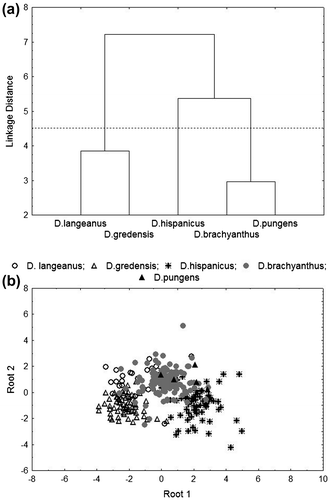
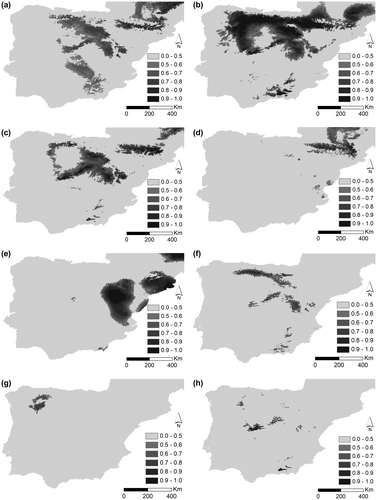
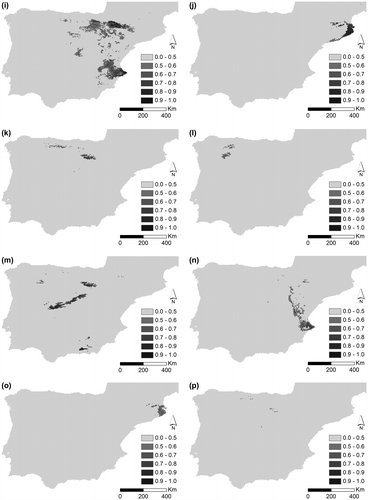
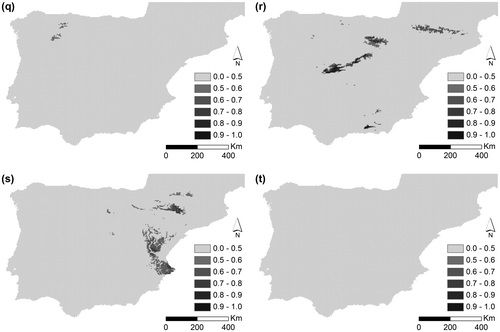
Figure 2. Potential distribution for (a) D. brachyanthus, (b) D. langeanus, (c) D. gredensis, (d) D. hispanicus and (e) D. pungens for LGM (approximately 22,000 years bc); under current conditions for (f) D. brachyanthus, (g) D. langeanus, (h) D. gredensis, (i) D. hispanicus; and (j) D. pungens; under RCP 4.5 future conditions scenario for (k) D. brachyanthus, (l) D. langeanus, (m) D. gredensis, (n) D. hispanicus; and (o) D. pungens; and under RCP 8.5 future conditions scenario for (p) D. brachyanthus, (q) D. langeanus, (r) D. gredensis, (s) D. hispanicus, and (t) D. pungens.
Table 1. The most discriminant morphological variables to distinguish the Iberian taxa of the D. pungens group (Fbial, minimum length of the epicalyx teeth, Fsab2 = maximum width of calyx – Crespí et al. Citation2007). The low F-values confirm the relevant morphological similarity between all subspecies.
Figure shows potential areas for LGM (a–d), current (e–h), and future scenarios (i–p) for the Iberian subspecies of D. pungens. The potential distribution area of D. hispanicus decreases under the LGM scenario, while in future scenarios (with increased temperature and lower precipitation) it increases significantly, clearly showing its preference for warmer and dryer habitats. Potential distribution of D. langeanus greatly decreases under higher temperatures and a drier climate (86 km2 for 8.5 2070). D. gredensis (1069 km2 for 8.5 2070) and D. hispanicus (1279 km2 for 8.5 2070) are the taxa that show the highest resilience responses to environmental variations, and therefore to climate change (once D. brachyanthus shows a decrease in future climate change scenarios – 33 km2 for 8.5 2070). Nonetheless, continuity analysis of overlapping areas according to taxon and environmental scenarios (Table ) clearly shows that D. brachyanthus and D. gredensis maintain an overlap between all taxa, except for D. pungens, which is predicted to lose all suitable potential habitat in future scenarios. In the RCP 8.5 scenario, only D. pungens is predicted to have no potential habitat area. The persistence of all taxa is guaranteed under smoother environmental conditions, here represented by the RCP 4.5 scenario.
Table 2. Overlapping values between subspecies for every scenarios (km2): First D. gredensis, and second D. brachyanthus, have the highest overlapping values for every scenario.
Environmental analysis by scenario and taxon for the abovementioned variables show important variations between all species. With the exception of D. pungens, with a very restricted potential habitat area predicted for current and future environmental conditions (even no representation for 8.5 2070 scenario), the potential habitat area predicted for D. brachyanthus and D. gredensis showed significant overlap with the other taxa for LGM and current scenarios. A significant restriction of potential area was observed in harsher scenarios for D. brachyanthus and D. langeanus, with the isolation of their populations and notorious environmental restrictions for their conservation.
The environmental variability analysis allows us to point to the warmest temperature of July (tmax7) and the lowest of June (tmin6), as well as average precipitation for the end of summer (average precipitation of September, prec9, and October, prec10) as the most discriminant environmental parameters to describe the dynamic of environmental change along the glacial and interglacial processes (Figure a and Table ). Elevation has no significant influence, since elevation ranges for each taxon were especially wide under LGM (as obtained for other genera – Benito Garzón, Sánchez de Dios, and Sáinz Ollero Citation2007 [Olalde et al. Citation2002]) and current scenarios (Crespí et al. Citation2007). For future scenarios, areas with higher elevation were modelled as having suitable potential habitat areas (Rocha et al. Citation2012; Silva et al. Citation2014). The mean values for the most discriminant variables per scenario are shown in Figure b. As expected, the LGM and future scenario 8.5 2070 reveal the most extreme values.
Figure 3. (a) DCA graphic representation for taxa analysed under LGM, current, and RCP 4.5 and 8.5 scenarios; and (b) average variation of the most discriminant variables per scenario (LGM, current and RCP 4.5 and 8.5), for all taxa analysed.
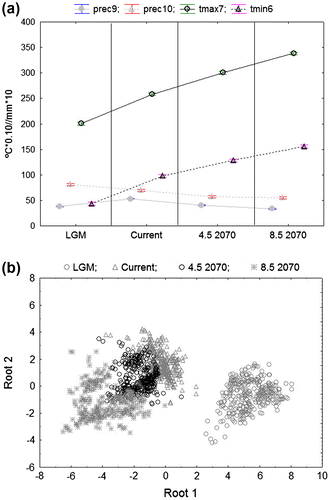
Table 3. Maximal temperatures for July, average precipitation for September and October, and minimal temperatures for June revealed the most discriminant environmental variables to distinguish the scenarios analysed (LGM, current conditions, and RCP 4.5, and 8.5 2070).
Discussion
When analysing taxa of the D. pungens group that occur in the Iberian Peninsula, two important patterns stand out under current environmental variability: two extreme patterns, one in the west (here represented by D. langeanus and D. gredensis) and the other in the east (D. pungens and D. hispanicus) of the Iberian Peninsula, as well as an intermediate and wider pattern (D. brachyanthus). The morphological study of these taxa have already revealed this phenomenon (Crespí et al. Citation2007), and better taxonomic designations have been suggested (D. pungens subsp. langeanus (Willk.) O. Bolòs & Vigo (Bolòs and Vigo Citation1974); D. pungens subsp. gredensis (Pau ex Caballero) A. Crespí, C. P. Fernandes, A. Castro, Bernardos & Amich (Crespí et al. Citation2007); D. pungens subsp. brachyanthus (Boiss.) Bernal, Fernández Casas, G. López, Laínz & Muñoz Garmendia (Bernal Citation1987); D. pungens subsp. hispanicus (Asso) O. Bolòs & Vigo (Bolòs and Vigo Citation1974)). In this regard, D. brachyanthus was clearly morphologically closer to eastern Dianthus individuals, although it was also the taxon closest to the morphological variability shown by D. langeanus and D. gredensis. These results agree with those found by Loidi et al. (Citation2015), since these species are concentrated in the central and northern mountain systems of the Iberian Peninsula (only D. brachyanthus and D. hispanicus are also present in the south-eastern mountains). Nevertheless, the potential behaviours shown for each of the scenarios here analysed reflect the potential of D. brachyanthus to biogeographically overlap all the other taxa. This phenomenon not only confirmed the largest morphological variability detected for this taxon (Crespí et al. Citation2007), but can also be understood as an effort to maintain the linkage with each other subspecies.
The insurance theory perspective and its resilience approach (Allison Citation2004) can be useful in explaining these results, as it could explain the persistence of the group D. pungens along the glacial–interglacial periods. In this sense, the morpho-environmental results show that D. gredensis is the most resilient taxon, based on the species’ environmental variability. This remarkable variability can be observed in the ability of this species to environmentally overlap all others (with the exception of D. pungens, which has no potential distribution for the 8.5 2070 scenario). Based on the same description as that applied by Rocha et al. (Citation2016) for genus Ononis L., the potential habitat distribution for D. gredensis persisted in the most restrictive scenarios (future scenarios, especially RCP 8.5), showing the persistence of this taxon. This result, and those found through the morphological approach exposed in Crespí et al. (Citation2007) and confirmed in the present study for all taxa of this group, suggest that there were no significant environmental differences between them in the past. At a morphological level, there is a remarkable overlap between the description of D. langeanus, D. gredensis and D. brachyanthus (Caballero López Citation1945): the apex of bracts is the only morphological trait used to distinguish D. langeanus with D. brachyanthus, as the differences between D. gredensis and D. brachyanthus are extremely subjective – pertaining to the shape of the calyx alone. For D. hispanicus, D. pungens and D. brachyanthus, the morphological differences are even more irrelevant – once again pertaining only to the shape of calyx and its length (Bernal, Laínz, and Garmendia Citation1990). On the other hand, the environmental characterization, interpreted from a resilience perspective, which is here understood as the amount of environmental responses that a taxon shows without changing into another species, distinguish two different trends. This discussion on resilience is based on Gunderson’s (Citation2000) concept of “the amount of disturbance that a system can absorb without changing state”. In this context, the western diversification of D. pungens group into D. gredensis, D. langeanus and D. brachyanthus morpho-environmental responses allowed its persistence along the western, northern and southern mountain systems, while the morphological and environmental overlap between D. hispanicus and D. pungens guaranteed its presence in the eastern of the Iberian Peninsula in the current scenario. The past (LGM) and future (4.5 2070 and 8.5 2070) scenarios do not distinguish these two tendencies: for LGM, those trends clearly overlapped, while for future climate changes significant fragmentation of the potential areas were observed. At the same time, the significant morphological overlap between all the taxa of this group confirms its possible consolidation as a taxonomic group.
In this sense, similar examples and descriptions have been reported. One example is the evolutionary description proposed by Valente et al. (Citation2010), in which there is the possibility of correlating the rapid response of the analysed taxa to environmental changes with the important ecological and geographical limitations to which it is exposed. According to the authors, such a condition also causes a phenomenon of explosive radiation, in which an initial genome could generate new morpho-genetic behaviours. Loidi et al. (Citation2015) provided comparable arguments, wherein the major irradiation observed in the Iberian mountain formations could be closely related to the ease of allopatric phenomena occurring by biogeographical isolation associated with these ecosystems. This process becomes ever more important as species become restricted to shorter elevation ranges (Li et al. Citation2014). Engler et al. (Citation2011) explained that climate change will have an even more decisive impact upon mountain and alpine ecosystems of southern Europe, especially due to thermophilization (Gottfried et al. Citation2012; Pérez-Obiol et al. Citation2011). In this context, our results confirm the significance of the average temperature increase, but also show how the elevation range associated with each of these taxa is a determinant of the higher resilience of the group. Here, it is also important to mention the binding effect, particularly that of the taxon D. gredensis. Both circumstances will not only ensure the persistence of all subspecies under the analysed scenarios, but will also allow the persistence of gene flow in between, thereby avoiding the potential effect of reproductive isolation. The effect of genetic flow in relation to the flora responses to climate change results are determinant, since it reduces the impact of marginal populations (Davis, Shaw, and Etterson Citation2005). However, the increased resilience against environmental changes (De Meaux and Mitchell-Olds Citation2003), and the ability to access a greater combination and diversity of micro refugees (Engler et al. Citation2011), were here confirmed for the Iberian taxa of the D. pungens group. Several other contributions reinforce the hypothesis of fragmentation and local refugees for mountain ecosystems (Diaz, Grosjean, and Graumlich Citation2003; Engler et al. Citation2009; Gottfried et al. Citation2012). Habitat structure transformations and replacement with generalist taxa will also be involved in these extremely rapid transformations, especially in mountain ecosystems (Moritz and Agudo Citation2013).
Based on these results, the hypothesis of a taxonomic group D. pungens for the taxa analysed is confirmed. In this sense, we propose the D. pungens group as the species and D. brachyanthus, D. langeanus, D. gredensis, D. hispanicus and D. pungens as their subspecies. The morpho-environmental characterization confirmed two opposite environmental trends (one for the western and northern, and the other for the eastern of the Iberian Peninsula) for current environmental conditions. Under this scenario, D. brachyanthus was confirmed as an intermediate morpho-environmental response between those opposite morpho-environmental behaviours. Nevertheless, the past and the future scenarios confirmed the consolidation of every taxa as part of the same group.
Notes on contributors
João Rocha, PhD biologist at the University of Porto (Portugal); modelling climate changing scenarios and sample collection.
Paulo Almeida, MsD ecologist at the University of Coimbra (Portugal); identification and sample collection.
Isabel Garcia-Cabral, MsD in environmental engineering at the University of Trás-os-Montes e Alto Douro (Portugal); sample collection.
Maxim Jaffe, environmental manager at the University of Cambridge (United Kingdom); discussion of results and development of manuscript.
Rubén Ramírez-Rodríguez, MsD biologist at the University of Salamanca (Spain); sample collection.
Francisco Amich, PhD biologist at the University of Salamanca (Spain); discussion of results and development of manuscript.
António L. Crespí, PhD biologist at the University of Salamanca (Spain); sample collection, discussion of results and development of manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Allison, G. 2004. “The Influence of Species Diversity and Stress Intensity on Community Resistance and Resilience.” Ecological Monographs 74: 117–134.10.1890/02-0681
- Benito Garzón, M., R. Sánchez de Dios, and H. Sáinz Ollero. 2007. “Predictive Modelling of Tree Species Distributions on the Iberian Peninsula during the Last Glacial Maximum and mid Holocene.” Ecography 30 (1): 120–134.10.1111/eco.2007.30.issue-1
- Bernal, M. 1987. “Notas sobre algunas especies del género Dianthus L. del NE de la Península Ibérica.” Anales del Jardín Botánico de Madrid 45: 363–367.
- Bernal, M., M. Laínz, and F. Garmendia. 1990. “Dianthus L.” In Flora Ibérica. Plantas Vasculares De La Península Ibérica E Islas Baleares [Flora Iberica. Flowering Plants of the Iberic Peninsula and Balearic Islands], edited by S. Castroviejo, M. Laínz, G. López González, P. Montserrat, F. Muñoz Garmendia, J. Paiva, and L. Villar, vol. 2, 426–462. Madrid: Real Jardín Botánico, C.S.I.C.
- Bhagwat, S.A., and K. J. Willis. 2008. “Species Persistence in Northerly Glacial Refugia of Europe: A Matter of Chance or Biogeographical Traits?” Journal of Biogeography 35: 464–482.10.1111/jbi.2008.35.issue-3
- Bolòs, O., and Vigo. 1974. “Notes sobre taxonomia i nomenclatura de plantes, I.” Butlletí de la Institució Catalana d´História Natural (Sec. Bot. 1) 38: 88.
- Brand, F. 2009. “Critical Natural Capital Revisited: Ecological Resilience and Sustainable Development.” Ecological Economics 68 (3): 605–612.10.1016/j.ecolecon.2008.09.013
- Caballero López, A. 1945. “Two Botanical Excursions in 1944.” Anales Del Jardín Botánico De Madrid 5: 505–521.
- Carvalho, M., J. Rocha, V. Carnide, S. Martins, M. Mus, F. Amich, R. Almeida, et al. 2016. “Biogeographic Divergences in the Iberian Flora. a Morpho-Anatomic, ISSR-Based, and Environmental Study of Iberian Buxus sempervirens L.” Turkish Journal of Botany 40: 1–16.10.3906/bot-1409-7
- Crespí, A. L., C. P. Fernandes, A. Castro, S. Bernardos, and F. Amich. 2007. “Morpho-Environmental Characterization of the Genus Dianthus (Caryophyllaceae) in the Iberian Peninsula: D. pungens Group.” Annales Botanici Fennici 44: 241–255.
- Davis, M. B., R. G. Shaw, and J. R. Etterson. 2005. “Evolutionary Responses to Changing Climate.” Ecology 86 (7): 1704–1714.10.1890/03-0788
- De Meaux, J., and T. Mitchell-Olds. 2003. “Evolution of Plant Resistance at the Molecular Level: Ecological Context of Species Interactions.” Heredity 91 (4): 345–352.10.1038/sj.hdy.6800342
- Diaz, H. F., M. Grosjean, and L. Graumlich. 2003. “Climate Variability and Change in High Elevation Regions: Past, Present and Future.” Climatic Change 59 (1): 1–4.10.1023/A:1024416227887
- Engler, R., C. F. Randin, P. Vittoz, T. Czáka, M. Beniston, N. E. Zimmermann, and A. Guisan. 2009. “Predicting Future Distributions of Mountain Plants under Climate Change: Does Dispersal Capacity Matter?” Ecography 32 (1): 34–45.10.1111/eco.2009.32.issue-1
- Engler, R., C. F. Randin, W. Thuiller, S. Dullinger, N. E. Zimmermann, M. B. Araújo, P. B. Pearman, et al. 2011. “21st Century Climate Change Threatens Mountain Flora Unequally across Europe.” Global Change Biology 17 (7): 2330–2341.10.1111/j.1365-2486.2010.02393.x
- Gómez, A., and D. H. Lunt. 2007. “Refugia within Refugia: Patterns of Phylogeographic Concordance in the Iberian Peninsula.” In Phylogeography of Southern European Refugia, edited by S. Weiss and N. Ferrand, 155–188. Dordrecht: Springer.10.1007/1-4020-4904-8
- Gottfried, M., H. Pauli, A. Futschik, M. Akhalkatsi, P. Barančok, J. L. B. Alonso, G. Coldea, et al. 2012. “Continent-Wide Response of Mountain Vegetation to Climate Change.” Nature Climate Change 2 (2): 111–115.10.1038/nclimate1329
- Gunderson, L. H. 2000. “Ecological Resilience in Theory and Application.” Annual Review of Ecology, Evolution and Systematics 31: 425–439.10.1146/annurev.ecolsys.31.1.425
- Hampe, A., J. Arroyo, P. Jordano, and R. J. Petit. 2003. “Rangewide Phylogeography of a Bird-Dispersed Eurasian Shrub: Contrasting Mediterranean and Temperate Glacial Refugia.” Molecular Ecology 12: 3415–3426.10.1046/j.1365-294X.2003.02006.x
- Hijmans, R. J., S. E. Cameron, J. L. Parra, P. G. Jones, and A. Jarvis. 2005. “Very High Resolution Interpolated Climate Surfaces for Global Land Areas.” International Journal of Climatology 25: 1965–1978.10.1002/(ISSN)1097-0088
- IPCC AR5 WG1. 2013. The Physical Science Basis. Working Group 1 (WG1) Contribution to the Intergovernmental Panel on Climate Change (IPCC). In Climate Change 20135th Assessment Report (AR5), edited by T. F. Stocker, D. Qin, G. -K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, and P. M. Midgley, 1535 pp. Cambridge: Cambridge University Press.
- Li, X. H., X. X. Zhu, Y. Niu, and H. Sun. 2014. “Phylogenetic Clustering and Overdispersion for Alpine Plants along Elevational Gradient in the Hengduan Mountains Region, Southwest China.” Journal of Systematics and Evolution 52: 280–288.10.1111/jse.v52.3
- Linares, J. C. 2011. “Biogeography and Evolution of Abies (Pinaceae) in the Mediterranean Basin: The Roles of Long-Term Climatic Change and Glacial Refugia.” Journal of Biogeography 38: 619–630.10.1111/jbi.2011.38.issue-4
- Loidi, J., J. A. Campos, M. Herrera, I. Biurrun, I. García-Mijangos, and G. García-Baquero. 2015. “Eco-Geographical Factors Affecting Richness and Phylogenetic Diversity Patterns of High-Mountain Flora in the Iberian Peninsula.” Alpine Botany 125 (2): 137–146.10.1007/s00035-015-0149-z
- Meinshausen, M., S. J. Smith, K. Calvin, J. S. Daniel, M. L. T. Kainuma, J.-F. Lamarque, K. Matsumoto, et al. 2011. “The RCP Greenhouse Gas Concentrations and Their Extensions from 1765 to 2300.” Climatic Change 109 (1-2): 213–241.10.1007/s10584-011-0156-z
- Moritz, C., and R. Agudo. 2013. “The Future of Species under Climate Change: Resilience or Decline?” Science 341 (6145): 504–508.10.1126/science.1237190
- Moss, R., M. Babiker, S. Brinkman, E. Calvo, T. Carter, J. Edmonds, I. Elgizouli, et al. 2008. “Towards New Scenarios for Analysis of Emissions, Climate Change, Impacts, and Response Strategies.” Edited by Intergovernmental Panel on Climate Change. Geneva.
- Olalde, M., A. Herrán, S. Espinel, and P. G. Goicoechea. 2002. “White Oaks Phylogeography in the Iberian Peninsula.” Forest Ecology and Management 156 (1-3): 89–102.10.1016/S0378-1127(01)00636-3
- Pérez-Obiol, R., G. Jalut, R. Julià, A. Pèlachs, M. J. Iriarte, T. Otto, and B. Hernández-Beloqui. 2011. “Mid-Holocene Vegetation and Climatic History of the Iberian Peninsula.” Holocene 21 (1): 75–93.10.1177/0959683610384161
- Picó, F. X., B. Méndez-Vigo, J. M. Martinez-Zapater, and C. Alonso-Blanco. 2008. “Natural Genetic Variation of Arabidopsis thaliana is Geographically Structured in the Iberian Peninsula.” Genetics 180 (2): 1009–1021.10.1534/genetics.108.089581
- Rocha, J., A. L. Crespí, M. García-Barriuso, G. Kozlowski, R. Almeida, J. Honrado, S. Talavera, and F. Amich. 2012. “Morpho-Environmental Characterization of the Genus Baldellia Parl. (Alismataceae) in the Iberian Peninsula, Balearic Islands and North Morocco.” Plant Biosystems 146 (2): 334–344.10.1080/11263504.2011.557096
- Rocha, J., R. Ramírez, M. Díaz, M. Martins, I. García-Cabral, F. Amich, R. Almeida, et al. 2016. “Morpho-Environmental Strategies in the Genus Ononis L. (Subsections Natrix and Viscosae) in Western Mediterranean.” Plant Biosystems. doi: 10.1080/11263504.2016.1244118.
- Rodríguez-Sánchez, F., A. Hampe, P. Jordano, and J. Arroyo. 2010. “Past Tree Range Dynamics in the Iberian Peninsula Inferred through Phylogeography and Palaeodistribution Modeling: A Review.” Review of Palaebotany and Palynology 162: 507–521.10.1016/j.revpalbo.2010.03.008
- Silva, R. A. D., J. Rocha, A. Silva, I. García-Cabral, F. Amich, and A. L. Crespí. 2014. “The Iberian Species of Scilla (Subfamily Scilloideae, Family Asparagaceae) under Climatic Change Scenarios in Southwestern Europe.” Systematic Botany 39 (4): 1083–1098.10.1600/036364414X682544
- Stringham, T. K., W. C. Krueger, and P. L. Shaver. 2001. “States, Transitions, and Thresholds: Further Refinement for Rangeland Applications.” Edited by Agricultural Experiment Station. Oregon: Oregon State University.
- Valente, L. M., G. Reeves, J. Schnitzler, I. P. Mason, M. F. Fay, T. G. Rebelo, M. W. Chase, and T. G. Barraclough. 2010. “Diversification of the African Genus Protea (Proteaceae) in the Cape Biodiversity Hotspot and beyond: Equal Rates in Different Biomes.” Evolution 64 (3): 745–760.10.1111/evo.2010.64.issue-3
- Weyant, J., C. Azar, M. Kainuma, J. Kejun, N. Nakicenovic, P. R. Shukla, E. La Rovere, and G. Yohe. 2009. “Report of 2.6 versus 2.9 Watts/M2 RCPP Evaluation Panel.” Edited by IPCC Secretariat. Geneva.