Abstract
Distinct distribution patterns were observed for several species of charophytes during a survey in Chile. We attempted to link these patterns to climatic conditions, but no direct causality between specific climatic factors and charophyte growth conditions could be identified. In this paper, correlations between water chemistry and occurrence of charophytes were tested as an alternative approach to explain the distribution pattern. Datasets with information about conductivity, alkalinity, pH and concentrations of calcium, magnesium, sodium, potassium, sulphate and chloride from sites inhabited by at least one charophyte species were analysed by principal component analysis. The results show that the distribution patterns of at least some species can be explained by niche differences with respect to water chemistry. The genera Chara and Nitella are in general well separated by their preferences for a given pH, alkalinity and conductivity range. Within the genus Chara, Chara squamosa, Chara braunii, Chara fulgens, and a yet unidentified taxon Chara sp., differ from this genus-specific pattern with respect to at least one parameter, whereas Chara vulgaris occupies a broad niche, covering almost the full range of all parameters except conductivity. Within the genus Nitella, niche separation by water chemistry was only possible between Nitella tenuissima and Nitella asagrayana. The chemical niche of Chara braunii was more closely related to the niche of the genus Nitella than the niche of other Chara species.
Introduction
The geographic distribution of species results from a number of determinants, which can be grouped into: (A) ecological processes (interactions between species and with abiotic factors) and (B) biogeographical processes (speciation, extinction and immigration of species) (Ricklefs and Schluter Citation1993). The metacommunity analysis sets out to resolve ecological processes (Chase and Leibold Citation2003) and spatial effects, resulting from species-specific dispersal rates, population size and connectivity (Hubbell Citation2001) in the observed distribution and association pattern (Cavalheri, Both, and Martins Citation2015; Hardy et al. Citation2012; Leibold, Economo, and Peres-Neto Citation2010).
To explain small- and mid-scale distribution pattern of charophytes, ecological processes are generally assumed to play a dominating role. Consequently, a number of bioindicators have been developed, with at least regional coverage (Kohler, Vollrath, and Beisl Citation1971; Krause Citation1981; Melzer Citation1999). All these systems presuppose that spatial effects are negligible, at least in restricted geographic scales, and that habitat filtering becomes the dominant process. The results of several studies (e.g. Cavalheri, Both, and Martins Citation2015) indicate that this assumption is valid only as long as environmental gradients are small. Leibold, Economo, and Peres-Neto (Citation2010) has proven the importance of both biogeographic history, as well as metacommunity processes for the regulation of zooplankton associations, even within a rather restricted range (lakes of the northeastern US), underlining the importance of environmental gradients for aquatic organisms.
In a recent survey of charophyte distribution along the Chilean climatic gradient, some species exhibited distinct distribution patterns, which could be correlated with bioclimatic zonation (Schubert et al. Citation2015). The parameters defining these bioclimatic zones, however, did not allow the identification of the underlying physiological mechanisms explaining these patterns. Frequency of thaw/freeze days or precipitation regime, both commonly applied to explain distribution patterns of terrestrial vegetation, are not useful parameters to explain the distribution of submerged aquatic organisms (Schubert et al. Citation2015). Water chemistry has a potentially high importance among possible alternative explanations for the observed distribution patterns, especially when environmental gradients are small with factors less important for submerged aquatic organisms (Schubert et al. Citation2015).
Macronutrient concentrations are potentially important charophyte water chemistry parameters, especially at low nutrient (particularly low phosphorus) concentrations (e.g. Corillion Citation1957; Krause Citation1997). On the other hand, field investigations show that charophytes also occur in lakes with high phosphorus concentrations, but are restricted to shallow water, suggesting light limitation rather than direct macronutrient effects (Blindow Citation1992; Portielje and Roijackers Citation1995; Schwartz and Hawes Citation1997). Comparatively little is known, however, about how water chemistry parameters, other than macronutrients, affect charophytes. Charophyte distribution and ion concentrations have been correlated in just a few local studies (see Doege et al. Citation2016). Strong links between water hardness and presence or absence of some species and whole genera have, however, been observed already. Migula (Citation1897: 90–91) seems to be the last author to deny the importance of calcium concentrations for charophytes.
The ability to turgor regulate may potentially explain the observed close correlation between ion concentrations and charophyte distribution. Charophytes have been shown to regulate their turgor in freshwater by maintaining constant internal potassium concentrations (Winter and Kirst Citation1990, Citation1991a, Citation1992). At higher salinities, a rather species-specific pattern of osmoregulation mechanisms supports ion regulation and keeps the turgor high enough to allow shoot elongation (Bisson and Kirst Citation1995). These mechanisms explain the differences in preferences for external ion concentrations among charophyte species; a well-established concept that allows us to distinguish freshwater, oligohaline, mesohaline and euryhaline species by their salinity acclimation ranges (Martin et al. Citation2004). At high conductivities, separation along the salinity range is expected to be of major importance, while other ion composition should play a subordinate role. At low conductivities, where osmoregulation plays a minor role, ion composition has a potentially high impact due to interference with potassium uptake (Beilby Citation2015; Bisson and Bartholomew Citation1984; Winter and Kirst Citation1991a).
Here, we investigate the influence of water chemistry on charophyte distribution by means of a set of more than 150 samples along the Chilean climatic and geochemical gradient.
Material and methods
Field work and study area
Field work was conducted during three expeditions in southern late summer (February to March) of 2011, 2012 and 2013 and one in early summer (November) of 2014. During these expeditions, the entire latitudinal gradient of macrobioclimatic zones of the Chilean mainland was sampled.
Sampling sites were pre-selected from satellite images (Google Earth). The main target was to get an overview of Chilean charophyte flora by sampling at least all large lakes accessible on Chilean mainland. Additionally, small water bodies, such as ephemeral ponds and creeks, were sampled along the route among the preselected sampling sites. This was done because some species prefer small or even ephemeral water bodies, which could not be traced by satellite images. Targeting on a floristic overview, all potential habitats for charophytes had to be taken into account. Therefore, even rice fields and ditches were sampled, when occurring along the route between the pre-selected large water bodies. Thus, most bioclimatic zones were sampled, except for the tundra-like cold-zone, an insular ecotone in southern Chile accessible only by boat (for further details see Schubert et al. Citation2015). Altogether more than 500 sites were investigated at least three times (samplings) by means of a double-fork (Krause Citation1997) for occurrence of submerged vegetation (if not immediately visible). Larger lakes were investigated at several locations along the shoreline; about 100 sites were sampled by snorkelling or SCUBA-diving. Charophytes were found at 318 sites. At sites with charophytes present, water samples were taken. Unfortunately, loss of water samples during transfer resulted in analysis of just 157 samples. Reasons for losses were leaking bottles, bottles breaking during off-road transfer and a complete loss of a parcel during transfer by post. However, all samples successfully transferred to the laboratory were analysed and the data-set presented here contains all samples of charophyte sites, where water samples were available. Irrespective of the large number of samples analysed, the data-set is limited by the lack of information about the tundra-like cold zone and by the loss of about half of the water samples. Thus, the present data-set is incomplete and contains only of data about occurrence of species, but cannot be used for identification of distributional limits of species.
Details of the full set of sites with charophyte distributions are published by Schubert et al. (Citation2015). An overview of the 157 sites for which water chemistry data are available is given in Appendix Table A1. All water samples were taken directly above the charophyte stands and stored refrigerated in darkness until analysis.
Water chemistry analysis
The conductivity was measured by HACH1Hq11d (Hach-Lange, Germany) conductivity meter and the pH by Multi3420 (WTW, Germany) pH-meter. Alkalinity was measured by the titration method, using bromocresol green indicator (APHA Citation2005).
For the quantitative analysis of the main cation and anion concentrations an ionchromatograph (Dionex ICS 3000) with suppressor technique for both ion types had been used. According to the lower level of quantification and the calibrated range, some samples with relatively low conductivity were measured undiluted. Samples with higher salinity were diluted up to 1:1000 with high purity water for the measurements. Successful participation in an annual inter-laboratory test, organized by r-concept (https://www.r-concept.de), proves our capacity to produce measurements of the appropriate resolution with the analytical methods applied (following EN ISO 14911:1999 and EN ISO 10304:2009–1). Measurements of test ion standards are part of the daily quality assurance procedure. Annual method validations determine lower level of quantification and prove the linearity of the calibration curve, as well as the homoscedasticity of the variance in the working range.
Data analysis
To calculate the quartiles shown in the box-whisker plots, the “quartile.ink”-functions of Excel2013 were used, giving the 25, 50 and 75% quartile values. Prior to analyses, auto-scaling (subtracting the mean value of the data-set from the data value, followed by division by the standard deviation of the data-set) and test for normal distribution were performed for all data available for the respective parameter, based on sites. Therefore, for sites where more than one charophyte species were present, the respective parameter was included into the auto-scaling procedure only once. Multidimenional scaling (MDS) and principal component analysis (PCA) were performed by means of the Primer6 program (Version 6.1.13; Primer-E Ltd.). MDS was based on a lower triangular resemblance matrix (Euclidean distance) of the sample parameters: pH; conductivity, alkalinity, Cl, SO4; Ca; K; Mg; Na as well as Na/K-ratio, Ca/Mg-ratio and SO4/Cl-ratio. Parameters chosen in primer 6 for MDS were: 25 repeats, minimum stress 0.01; Kruskal fit scheme 1. For the 2D graph, shown in Figure , the maximum number of iterations was not used and the minimum stress of 0.06 occurred 18 times out of 25 repetitions of the iteration process.
Results
Box-whisker graphs are shown for latitudinal ranges and water chemistry parameters of charophyte sites (Figures ). The values for Chara spp. and Nitella spp. are calculated for all species belonging to the genus, independently of the number of sites, but only species with at least three datasets for the parameter in question are shown in the Figures .
Figure 1. Box-whisker plot of latitudinal distributions of all Chara- and Nitella-sites included in the water chemistry dataset. Minimum, maximum and median latitude are given as well as 50% and 75% quartiles. Number of sites are given in brackets; only species with at least 5 sites are shown; genus Chara marked in grey. Please note that direction North is at low absolute numbers of latitude.
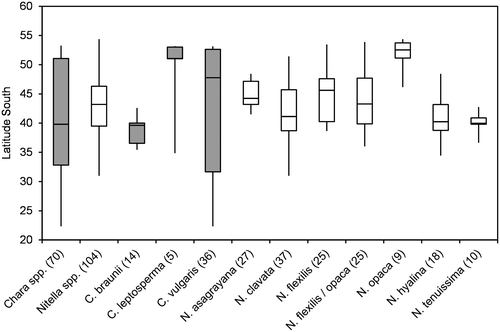
Figure 2. Box-Whisker plot of pH values for taxa with at least 3 values in the water chemistry dataset. Minimum, maximum and median pH are given as well as 50% and 75% quartiles (boxes). Number of values are given in brackets; Chara spp. marked in grey.
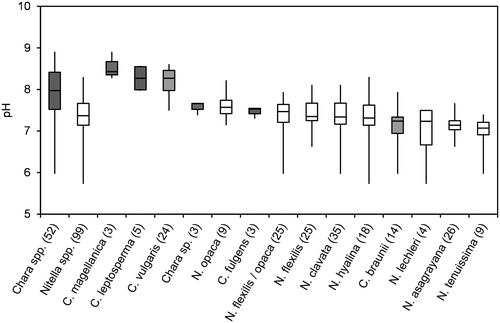
Figure 3. Box-Whisker plot of conductivity values (log scale) for all taxa with at least 3 values in the water chemistry dataset. Minimum, maximum and median values are given as well as 50% and 75% quartiles (boxes). Number of values are given in brackets; Chara spp. marked in grey.
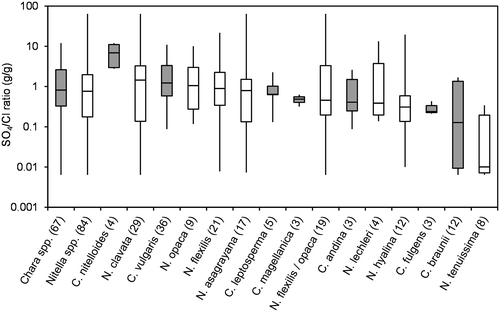
Figure 4. Box-Whisker plot of alkalinity values for all taxa with at least 3 values in the water chemistry dataset. Minimum, maximum and median values are given as well as 50% and 75% quartiles (boxes). Number of values are given in brackets; Chara spp. marked in grey.
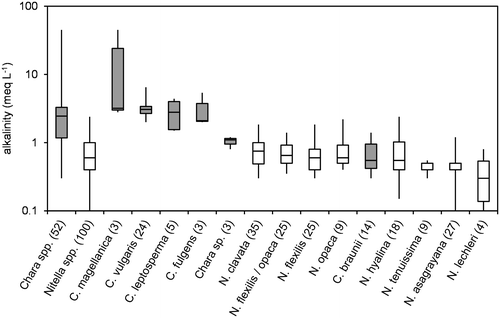
Figure 5. Box-Whisker plot of Na/K-ratios (g/g) for all taxa with at least 3 values in the water chemistry dataset. Minimum, maximum and median values are given as well as 50% and 75% quartiles (boxes). Number of values are given in brackets; Chara spp. marked in grey.
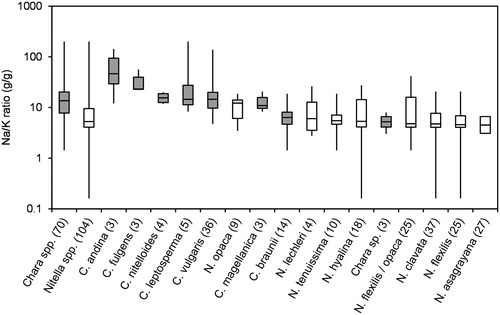
Figure 6. Box-Whisker plot of Ca/Mg-ratios (g/g) for all taxa with at least 3 values in the water chemistry dataset. Minimum, maximum and median values are given as well as 50% and 75% quartiles (boxes). Number of values are given in brackets; Chara spp. marked in grey.
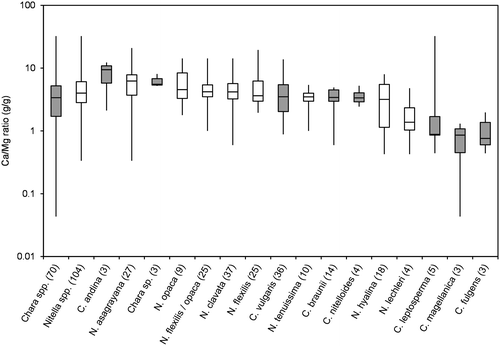
Figure 7. Box-Whisker plot of SO4/Cl-ratios (g/g) for all taxa with at least 3 values in the water chemistry dataset. Minimum, maximum and median values are given as well as 50% and 75% quartiles (boxes). Number of values are given in brackets; Chara spp. marked in grey.
Because water chemistry data were not available from all the sites sampled by Schubert et al. (Citation2015), regional coverage of the data-set had to be tested first. Figure shows the latitudinal distribution of all charophyte sites, where the water chemistry was analysed. The same general pattern was found, as described by Schubert et al. (Citation2015). Nitella spp. are more frequent in the southern part of Chile, while Chara spp. are more frequent at the lower latitudes. Chara vulgaris L. the most frequent Chara-species, covers the full latitudinal range sampled. Chara leptosperma A. Braun occupies the southernmost part of the area investigated, but has one northern location as well. Nitella opaca (C. Agardh ex Bruzelius) C. Agardh differs from N. flexilis (L.) C. Agardh by a more southern distribution.
Figure gives an overview over pH-ranges. In general, Chara spp. were found in water bodies with higher pH than Nitella spp. However, some Chara species (Chara sp., C. fulgens Filarsky and C. braunii Gmelin) were most frequent at low pH preferred by Nitella spp. Nitella species often covered a rather large range of pH-values, while Chara species, again except of C. braunii, were restricted to more narrow pH-ranges. The large pH-range for the genus Chara (Chara spp.) is explained by the fact that Chara braunii prefers pH conditions in a similar range as Nitella spp.
Figure summarizes the conductivity measurements. Nitella spp. prefer sites with lower conductivity than Chara spp. However, Chara vulgaris, the most frequent Chara species, and C. andina (A.Br.) R.D.W. were found in almost the full range of conductivities. The highest salinities were recorded for Chara fulgens (> 20 mS cm−1) and in one of the three sites of Chara magellanica (A.Br.) R.D.W. (>18 mS cm−1).
A pattern similar to conductivity can be observed for alkalinity (Figure ). Chara spp. prefer higher alkalinities than Nitella spp. There is little overlap between the genera, apart from the fact that Chara braunii and Chara sp. are found within the range of alkalinity values typical for Nitella spp.
Because ion regulation has been shown to be a crucial factor for control of osmotic potential (e.g. Bisson and Kirst Citation1995; Winter and Kirst Citation1991a, Citation1991b; Winter, Soulié-Märsche, and Kirst Citation1996), ion ratios are shown instead of absolute ion concentrations (Figures ). Figure , and show Na/K, Ca/Mg and SO4/Cl ratios, respectively, for all taxa with at least three datasets. No distinct differences between the genera can be seen for the three ratios. The slightly higher median of Na/K-ratios for Chara spp. arise from the salinity tolerance of species Chara fulgens and Chara andina. The same range, however, is covered by some of the Nitella species (see the Nitella spp. calculated from all Nitella sites and including the data of species with less than three occurrences successfully sampled and analysed).
In order to get an overview of the pattern of species presence at a specific set of water chemistry conditions, multidimensional scaling (MDS) based on all parameters and all sites was performed (Figure ). The outliers in the upper half of the upper panel are sites of Chara leptosperma A. Braun and cf. Tolypella glomerata (Desv.) Leonh. (left top, CL14/CL15), Chara fulgens (middle top, CL252) and one of the sites of Chara magellanica (right top, CL54).
Figure 8. Multidimensional plot of water chemistry data. Upper panel: MDS plot of the full set of water chemistry data (for details see material and methods); lower panel: zoom of the central part of the MDS-plot.
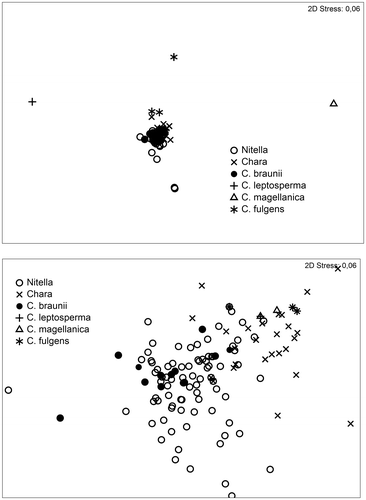
As can be seen from Table 1, the above-mentioned sites are somewhat unusual with respect to water chemistry and would consequently influence any factorial analysis to an extent masking potential differences between the remaining sites. The C. magellanica site in question (CL54) is distinct with respect to extreme alkalinity. All three C. fulgens samples (CL252, CL245, CL 246) are distinguished from the rest by an extreme ion concentrations, whereas CL14/CL15 (C. leptosperma and cf. Tolypella glomerata) exhibit an extreme Na/K-ratio of 200 (the next ratios in line are 142, 136, 99 and 56, while all other Na/K ratios are below 50). These five sites are therefore excluded from the next steps of analysis.
The lower panel of Figure shows a zoom into the center part of the upper panel. Chara spp. and Nitella spp. are rather well separated, except for Chara braunii, which is placed in the middle of the Nitella spp.-part of the plot.
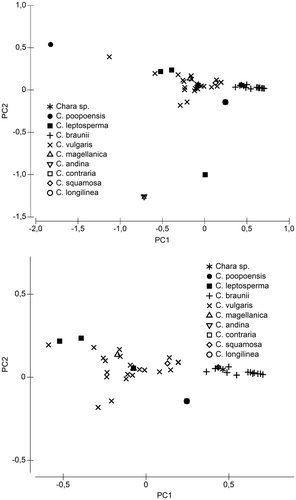
While the factors responsible for these differences between Chara spp. and Nitella spp./Chara braunii are obvious already from Figures , differences within these groups require more detailed analysis. Thus, principal component analysis of a subset of the data, containing only Chara spp. (apart from the sites of Chara fulgens, C. leptosperma and C. magellanica mentioned above) was performed. In the first run, analysing all sites with Chara spp. for pH; conductivity, alkalinity, Cl, SO4; Ca; K; Mg and Na, pH was the dominant factor (not shown). However, because pH may change in day/night cycles to a degree influenced by alkalinity (which is part of the data-set anyway) and eutrophication (which is not in the target of the study) and therefore may reflect sampling time rather than average conditions, this factor was excluded in a second run (Figure ). But even if pH is excluded from the analysis, Chara braunii is still distinctly separated from the other Chara species (Figure ). Only the sites of the Chara sp. are located within the range of Chara braunii. The pattern in Figure is dominated by alkalinity, receiving an Eigenvector value of −0.946 for the first axis (See Supplementary Table 1).
Figure 9. Principal component analysis of a subset of water chemistry data, containing only data about sites with occurrence of Chara-species. The lower panel zooms for better visibility into the centre region, where all C. braunii sites are located. For details of the PCA see Supplementary Table 1.
Principal component analysis of a similar subset of data, containing all Nitella sites and the sites of Chara braunii, revealed no distinct pattern (See Supplementary Figure 1, and Supplementary Table 2). The contribution of all factors analysed on the first axis and explaining 58% of the variance, is almost equal, ranging from −0.438 (magnesium) to −0.371 (sulphate). Alkalinity dominates the second axis with −0.674, which explains another 18% of the variance (See Supplementary Table 2). In contrast to Chara spp. (Figure ), Nitella spp. together with Chara braunii form a homogeneous group without any distinct separations.
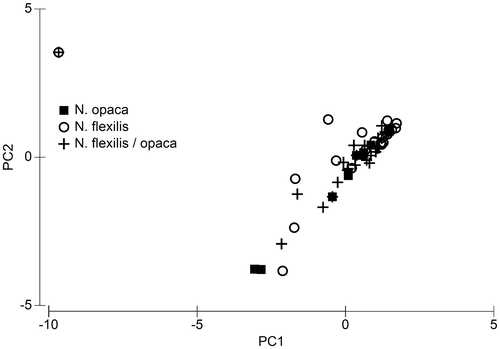
As far more data per species are available for some Nitella spp., compared to Chara spp., some specific questions about distribution patterns might be answered by testing the subsets. Figure shows the result of a principal component analysis of all data of Nitella opaca and N. flexilis (see also Supplementary Table 3). No differences in water chemistry between sites, occupied by these species, could be observed. The data from both species and from infertile and therefore difficult to classify specimens (N. flexilis/opaca) scattered without forming distinct clusters.
Figure 10. Principal component analysis of a subset of water chemistry data, containing only data from sites with occurrence of Nitella opaca, Nitella flexilis and sterile specimen which belong either to N. flexilis or N. opaca (N. flexilis / opaca). For details of the PCA see Supplementary Table 3.
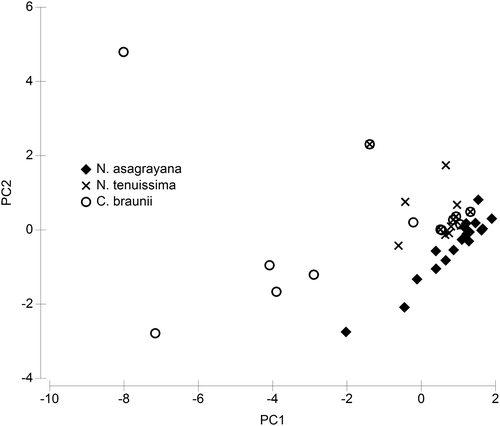
In contrast, Nitella asagrayana Schaffner ex Nordstedt and N. tenuissima (Desvaux) Kützing are distinctly separated (Figure ). C. braunii, which is included in this analysis, overlaps exclusively with N. tenuissima. The separation is explained by a preference for higher conductivities and magnesium concentrations (−0.450 and −0.439, respectively, on the first axis with an Eigenvalue of 4.59, explaining 57% of the variance, See Supplementary Table 4) of N. tenuissima and, even more pronounced, C. braunii. On the second axis (Eigenvalue 1.56, explaining 20% of the variance, See Supplementary Table 4), calcium concentrations dominate by −0.527, supporting the separation already initiated by the first axis.
Discussion
The data-set presented here is one of the most comprehensive sets on water chemistry data for charophytes worldwide. All 157 datasets were obtained by a uniform methodology for analysis of water chemistry, which is a fundamental prerequisite for direct comparison (Doege et al. Citation2016). This data-set covers nearly half of the charophyte sites known for Chile (Schubert et al. Citation2015). The geographical pattern of occurrence of species and genera, as described by Schubert et al. (Citation2015), is still retained in this data-set (Figure ), so analysis for correlation of the observed patterns due to water chemistry parameters should be possible.
However, the data-set provides only an approximation of conditions that charophytes can be found in, which may differ from the conditions required for germination or formation of oospores for instance. Water chemistry may vary on different time scales relevant to charophytes, ranging from day/night variations in pH by photosynthesis and respiration, occasional precipitation-dependent dilution by rainwater and seasonal rhythms related to temperature-dependent erosion intensity and precipitation regime in the catchment area. In general, it might be assumed that variability in water chemistry increases with decreasing volume of the respective water body, but this involves only to the rate of change, not to the amplitude, because even large lakes in dry subtropical or subantarctic regions have been shown to be ephemeral. So the following analysis, based on single sampling, gives a snapshot information, not reflecting the conditions necessary for establishment of stable charophyte distributions.
Generally, Nitella species were found at lower values of pH, conductivity and alkalinity than Chara species. Krause (Citation1997) and Corillion (Citation1957), however, mentioned some overlap in this pattern. For instance, Nitella hyalina (DC.) C. Agardh was found at pH as high as 8, while Chara braunii occurred at pH lower than 6. Nitella asagrayana and, to a lesser extent N. hyalina, were able to tolerate high conductivities, while the alkalinity pattern was more consistent. Nitella spp. were rarely found at alkalinities above 2 meq L−1, which is the lower limit of the 50% quartile of Chara spp. Just one site of N. hyalina and two sites of N. opaca had alkalinities above 2 meq L−1, while two Chara species, C. braunii and Chara sp., were only found at alkalinities below 2 meq L−1. A similar pattern was observed for Na/K ratios, where C. braunii and Chara sp. again were more closely related to Nitella spp. SO4/Cl-ratios and Ca/Mg-ratios did not distinctly differ among genera or species. Absolute concentrations of these ions may be more important parameters.
When combining all water chemistry data, Chara spp. and Nitella spp. are not well separated, mainly because C. braunii fully overlaps with Nitella spp. In a subset of data consisting of Chara spp. only, the separation of C. braunii from the other species of this genus becomes even more obvious and is explained by the preference of this particular Chara-species for low pH and low alkalinity sites. This striking similarity between Chara braunii and Nitella spp. provokes speculations about morphological factors: Could there be a direct link between lack of cortication and preference for low pH and low alkalinity conditions? Our own data-set shows that this is not a general rule. C. fulgens, also belonging to the subsection Charopsis (sensu Wood Citation1962), is separated from both C. braunii and Nitella spp. by its high habitat salinities, occurs at similar pH conditions as Nitella spp., while the alkalinities of the C. fulgens sites are clearly above the range of Nitella spp. and located in the 75% quartile of Chara spp. On the other hand, Chara sp., which is a corticated (see Blindow et al. submitted), exhibits a similar niche structure as C. braunii. A direct and general link between cortication and preference for low pH/low alkalinity conditions is therefore unlikely. In his definition of charophyte communities, Krause (Citation1997) combined C. braunii as a species typical for low (subneutral) pH conditions with several Nitella spp. (e.g., N. flexilis). A Nitella community consisting of, for instance, N. mucronata (A. Braun) F. Miquel and N. tenuissima was described as typical for neutral pH conditions, while a community of Chara spp. for alkaline conditions.
Within the genus Nitella, two phenomena were of special interest: (A) the more southern occurrence of N. opaca, compared to N. flexilis and, (B) the striking geographic separation of N. tenuissima and N. asagrayana within a single bioclimatic zone (Schubert et al. Citation2015).
No differences in chemical parameters were found between Nitella flexilis and N. opaca. The preference of N. opaca for higher latitudes can therefore not be explained by water chemistry parameters. A similar preference of N. opaca for higher latitudes has also been found for the northern hemisphere, where the species is very common in the northernmost parts of Scandinavia (Langangen Citation2007). It is possible that the latitudinal difference between N. flexilis and N. opaca arises from the ability of N. opaca to complete its annual growth cycle within a shorter period, allowing reproduction at higher latitudes (Becker and Wolff Citation2016). There are similar observations from central Europe, where N. opaca is characterized as a “spring species” (e.g. Becker and Wolff Citation2016; Krause Citation1997).
Sites of Nitella asagrayna and N. tenuissima are rather well separated by water chemistry characteristics with higher conductivities and magnesium concentrations in sites of N. tenuissima. This finding supports the assumption that the geographic separation between the two Nitella species is caused by differences in their chemical niche requirements. Chara braunii, a species often co-occurring with N. tenuissima, differs from N. tenuissima only by occurring also at higher conductivities and Mg concentrations, where N. tenuissima were not found.
Some unexpected findings arise from the data. The first and probably most striking discovery is that N. asagrayana, N. hyalina and N. clavata were found at conductivities some way above the salinity limit of 1.5 PSU, given for Nitella spp. by Winter and Kirst (Citation1991b). Thus the genus Nitella can regulate turgor as well as the internal osmotic pressure (for a review, see Beilby Citation2015 and references therein). This assumption is supported by the high Na/K-ratios measured at sites with Nitella occurrences.
In the genus Chara, C. vulgaris covers an extraordinarily broad range of water chemistry conditions, including conductivity. This species is described as oligohaline-halotolerant by several authors, and salinity acclimation has been studied thoroughly (e.g. Winter and Kirst Citation1990, Citation1991b). The large conductivity range of this species may provoke doubts about its taxonomic classification in Chile. However, the upper limit corresponds to a salinity of about 6 PSU, which is close to the limit reported from European occurrences (e.g. Corillion Citation1957; upper salinity limit of 5 PSU). In contrast, C. magellanica was found at salinities up to 12, far above the limits of C. vulgaris. Our ecological data thus support the taxonomic decision to treat Chara magellanica, originally described by Braun (Braun and Nordstedt Citation1882) as a variety of the freshwater species C. papillosa Kützing (syn. C. intermedia, see Schubert, Blindow, and van de Weyer Citation2016), as a separate species (see Blindow et al. submitted).
Conclusion
In conclusion, the results presented here demonstrate, that ecological niches of single charophyte species cannot be separated by water chemistry data alone. In some cases, such as N. tenuissima and N. asagrayana, strong preferences may be explained by differences in water chemistry between geographically separated regions. Chara braunii and the unidentified Chara sp. were more related to Nitella spp. than to Chara spp. with respect to the full set of parameters available. For sites of a number of other frequently occurring species, however, the parameters measured are not very different. An exception is conductivity, clearly indicating the preference of C. fulgens for sites with high salinity. Strong indications for the presence of turgor regulation in some Nitella species require further physiological investigation.
Notes on contributors
Hendrik Schubert is a marine biologist specialised in aquatic ecology and working at the University of Rostock. Contribution: general concept and research question, field work, data analysis, interpretation of results, manuscript preparation.
Irmgard Blindow is a limnologist and head of the Biological Station of the University of Greifswald. Contribution: general concept and research question, field work, interpretation of results, manuscript preparation.
Dirk Schories is a marine biologist, investigating marine biodiversity of the Chilean coast as a DAAD fellow at the Universidad Austral de Chile in Valdivia. Contribution: general concept and research question, field work, interpretation of results, manuscript preparation.
Margarete Mages is a biotechnologist specialised on element analytics at the Helmholtz Centre for Environmental Research. Contribution: element analytics, data analysis, interpretation of results, manuscript preparation.
Wolf von Tümpling is a chemist specialised on water analytics and environmental chemistry; head of central water analytics and chemometrics at the Helmholtz Centre for Environmental Research. Contribution: element analytics, data analysis, interpretation of results, manuscript preparation.
Stefan Woelfl is a limnologist at the Instituto de Ciencias Marinas and Limnologicas Universidad Austral de Chile in Valdivia. Contribution: general concept and research question, element analytics, data analysis, interpretation of results, manuscript preparation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the German academic exchange program DLR-IB/CONICYT common grant 01DN12026 CHL 2009-182.
Supplemental data
Supplemental data for this article can be accessed at https://doi.org/10.1080/23818107.2017.1370612
TABG_1370612_Supplementary_Material.docx
Download MS Word (217.6 KB)Acknowledgments
We thank Ronny Marquardt for valuable help during the field work. The authors want to thank Mary Beilby for English correction and the two anonymous reviewers for comments and amendments.
References
- APHA. 2005. Standard Methods for the Examination of Water and Waste Water. 21st ed. Washington, DC: American Public Health Association.
- Becker, R., and P. Wolff. 2016. “12.29 Nitella opaca.” In Armleuchteralgen - Die Characeen Deutschlands [Stoneworts – The Charophytes of Germany], edited by A. G. Characeen Deutschlands, 455–469. Berlin: Springer Verlag.
- Beilby, M. J. 2015. “Salt Tolerance at Single Cell Level in Giant-Celled Characeae.” Frontiers in Plant Science 6: 226. fpls.2015.00226.
- Bisson, M. A., and D. Bartholomew. 1984. “Osmoregulation or Turgor Regulation in Chara?” Plant Physiology 74: 252–255.10.1104/pp.74.2.252
- Bisson, M. A., and G. O. Kirst. 1995. “Osmotic Acclimation and Turgor Pressure Regulation in Algae.” Naturwissenschaften 82: 461–471.10.1007/BF01131597
- Blindow, I. 1992. “Long and Short Term Dynamics of Submerged Macrophytes in Two Shallow Eutrophic Lakes.” Freshwater Biology 28: 15–27.10.1111/fwb.1992.28.issue-1
- Blindow, I., R. Marquardt, D. Schories, and H. Schubert. Forthcoming. “Charophyceae of Chile – Taxonomy and Distribution. Part 1. Subfamily Chareae.” Submitted to Nova Hedwigia.
- Braun, A., and O. Nordstedt. 1882. “Fragmente einer Monographie der Characeen. Nach den hinterlassenen Manuskripten A. Braun’s herausgegeben von Dr. Otto Nordstedt [Fragments of A Monograph of the Characeae. Basing on Manuscripts of the Late A. Braun edited by Dr. Otto Nordstedt].” Abhandlungen der königlichen Akademie der Wissenschaften zu Berlin 1882: 1–211, pl. 1-7.
- Cavalheri, H., C. Both, and M. Martins. 2015. “The Interplay between Environmental Filtering and Spatial Processes in Structuring Communities: The Case of Neotropical Snake Communities.” PLoS ONE 10 (6): e0127959.10.1371/journal.pone.0127959
- Chase, J. M., and M. A. Leibold. 2003. Ecological Niches: Linking Classical and Contemporary Approaches: Interspecific Interactions. Chicago, IL: University of Chicago Press.10.7208/chicago/9780226101811.001.0001
- Corillion, R. 1957. Les charophycées de France et d’Europe Occidentale [The Charophytes of France and Western Europe]. Réimpression autorisée 1972. Koenigstein: Otto Koeltz Verlag.
- Doege, A., K. van de Weyer, R. Becker, and H. Schubert. 2016. “Bioindikation mit Characeen.” In Armleuchteralgen - Die Characeen Deutschlands [Bio-Indication with Charophytes. In: Stoneworts – The Charophytes of Germany], edited by A. G. Characeen Deutschlands, 97–138. Berlin: Springer Verlag.
- Hardy, O. J., P. Couteron, F. Muñoz, B. R. Ramesh, and R. Pélissier. 2012. “Phylogenetic Turnover in Tropical Tree Communities: Impact of Environmental Filtering, Biogeography and Mesoclimatic Niche Conservatism.” Global Ecology and Biogeography 2012: 1007–2016.
- Hubbell, S. P. 2001. The Unified Neutral Theory of Biodiversity. Princeton: Princeton University Press.
- Kohler, A., H. Vollrath, and E. Beisl. 1971. “Zur Verbreitung, Vergesellschaftung und Ökologie der Gefäss-Makrophyten im Fliessgewässer Moosach (Münchener Ebene) [Distribution, Sociology and Ecology of Higher Plants in River Moosach (Munich plateau)].” Archiv für Hydrobiologie 69 (333): 365.
- Krause, W. 1981. “Characeen als Bioindikatoren für den Gewässerzustand [Charophytes as Bio-Indicators of Water Status].” Limnologica 13 (399): 418.
- Krause, W. 1997. Charales (Charophyceae): Süsswasserflora von Mitteleuropa Band 18 [Freshwaterflora of Central Europe, Volume 18]. Jena: Gustav Fischer Verlag.
- Langangen, A. 2007. Charophytes of the Nordic Countries. Oslo: Saeculum ANS.
- Leibold, M. A., E. P. Economo, and P. Peres-Neto. 2010. “Metacommunity Phylogenetics: Separating the Roles of Environmental Filters and Historical Biogeography.” Ecology Letters 2010: 1290–1299.
- Martin, G., K. Torn, I. Blindow, H. Schubert, R. Munsterhjelm, and C. Henricson. 2004. “Introduction to Charophytes.” In Charophytes of the Baltic Sea, edited by H. Schubert and I. Blindow, 3–14. Königstein: Gantner Verlag.
- Melzer, A. 1999. “Aquatic Macrophytes as Tools for Lake Management.” Hydrobiologia 395–396: 181–190.10.1023/A:1017001703033
- Migula, W. 1897. Die Characeen Deutschlands, Oesterreichs und der Schweiz [The Charophytes of Germany, Austria and Switzerland]. 2nd ed. Leipzig: Eduard Kummer Verlag.
- Portielje, R., and R. M. M. Roijackers. 1995. “Primary Succession of Aquatic Macrophytes in Experimental Ditches in Relation to Nutrient Input.” Aquatic Botany 50 (127): 140.
- Ricklefs, R. E., and D. Schluter. 1993. Species Diversity in Ecological Communities: Historical and Geographical Perspectives. Chicago, IL: University of Chicago Press.
- Schubert, H., R. Marquardt, D. Schories, and I. Blindow. 2015. “Biogeography of Chilean charophytes.” Aquatic Botany 120: 129–141.10.1016/j.aquabot.2014.06.005
- Schubert, H., I. Blindow, and K. van de Weyer. 2016. “12.14 Chara papillosa.” In Armleuchteralgen - Die Characeen Deutschlands [Stoneworts – The Charophytes of Germany], edited by A. G. Characeen Deutschlands, 318–328. Berlin: Springer Verlag.
- Schwartz, A. M., and I. Hawes. 1997. “Effects of Changing Water Clarity on Characean Biomass and Species Composition in A Large Oligotrophic Lake.” Aquatic Botany 56 (169): 181.
- Winter, U., and G. O. Kirst. 1990. “Salinity Response of A Freshwater Charophyte, Chara vulgaris.” Plant, Cell and Environment 13 (123): 134.
- Winter, U., and G. O. Kirst. 1991a. “Partial Turgor Pressure Regulation in Chara canescens and its Implications for A Generalized Hypothesis of Salinity Response in Charophytes.” Botanica Acta 104: 37–46.10.1111/(ISSN)1438-8677b
- Winter, U., and G. O. Kirst. 1991b. “Vacuolar Sap Composition During Sexual Reproduction and Salinity Stress in Charophytes.” Bulletin de la Société Botanique de France 138 (85): 93.
- Winter, U., and G. O. Kirst. 1992. “Turgor Pressure Regulation in Chara aspera (Charophyta): The Role of Sucrose Accumulation in Fertile and Sterile Plants.” Phycologia 31 (240): 245.
- Winter, U., I. Soulié-Märsche, and G. O. Kirst. 1996. “Effects of Salinity on Turgor Pressure and Fertility in Tolypella (Characeae).” Plant, Cell and Environment 19: 869–878.10.1111/pce.1996.19.issue-7
- Wood, R. D. 1962. “New Combinations and Taxa in the Revision of Characeae.” Taxon 11 (7): 25.
Table 1. Overview about sites and water chemistry data used. Missing sample numbers are due to sites without charophyte occurrence.