Abstract
Chara canescens lives in shallow brackish waters in the northern hemisphere. It has been described as an annual species that mostly reproduces by parthenogenesis, being the only species of Chara able to do so. However, much is still unknown about its life cycle and the differences between the sexual and the parthenogenetic populations. We monitored the life cycle of a parthenogenetic population growing in a brackish interdunal pond in the Albufera de València Natural Park (Spain). Approximately 30 specimens were harvested every 15–30 days throughout 2015. The longest polar axis and the largest equatorial diameter were measured, and the isopolarity index was calculated for the oogonia and the oospores. The non-swollen and the swollen oogonia were distinguished by size. This population had a short life cycle, but two types of shoots with different life history traits were found. A few small individuals overwintered underneath the emergent vegetation. In early March, new shoots appeared from the germinating oospores, growing fast and colonizing more open waters. The first oogonia appeared in April, enlarging and ripening along the first five whorls of the shoots. The oospores were produced in less than 15 days after the first oogonia emergence. The ripe oospores were small (average size ± SD: 392 ± 34 × 219 ± 24 μm; isopolarity index: 181 ± 22). Parthenogenetic reproduction lasted 5 months. No new fructifications were produced from September onwards. Although the oospores were smaller than those described in the literature for the species, they were clearly viable and enabled the persistence of the population through the winter.
Introduction
Temporal variations in plant populations are tightly linked to the environmental conditions and depend on the phenology of species (i.e. the timing of the different development stages in their life cycle; Forrest and Miller-Rushing Citation2010). The seasonality of the growth and reproduction, by means of vegetative or sexual propagules, determine the population dynamics, the reproductive success and the persistence of species. Particularly in those inland aquatic ecosystems subjected to drought or eutrophication, the long-term permanence of submerged macrophytes relies on the production of propagules that can resist such environmental disturbances (Brock and Casanova Citation1991). For charophytes (green macroalgae), the production of the oospores guarantees the survival of populations, even in habitats that have been negatively affected for several years (Rodrigo and Alonso-Guillén Citation2013; Rodrigo, Alonso-Guillén, and Soulié-Märsche Citation2010). Under the current climate warming scenario, charophytes stands from the Mediterranean shallow ecosystems are especially vulnerable to desiccation. Projections point to altered precipitation regimes and higher temperatures in the region that will lead to lower water levels in inland aquatic habitats and, eventually, will turn many permanent shallow systems into temporary ones (IPCC Citation2014). Faced with this situation, our understanding of the timing of charophyte development can help us to predict the future of these populations. Until recently, just a few studies focused on the life cycle and the temporality of sexual reproduction in charophytes (Bonis et al. Citation1993; Brock and Casanova Citation1991; Casanova Citation1994; Casanova and Brock Citation1999). However, and particularly for the annual species, the timing of germination and the production of the oospores for the storage in the propagule bank determine the population dynamics.
Among the annual species, Chara canescens Loiseleur (Charales) lives throughout the northern hemisphere, being the only European charophyte to have haplostichous cortication and separate sexes (dioecious). It is an uncommon species: the only known species of the genus Chara able to produce oospores by parthenogenesis (without fertilization; Ernst 1918 as quoted in Braun et al. [Citation2007]). In fact, most of the populations in Europe do not contain any male shoots and consist of diploid parthenogenetic females only (Schaible, Bergmann, and Schubert Citation2011). It is possible to differentiate between the females from the sexual and the parthenogenetic populations genetically, but no distinguishing morphological traits have been found (Schaible et al. Citation2009). C. canescens is a small to medium-sized charophyte with a rigid axis covered by well-developed and clustered spine-cells that give it a brush-like appearance (Cirujano et al. Citation2008; Krause Citation1997; Mouronval et al. Citation2015; Urbaniak and Gąbka Citation2015). The species is easy to recognize because of its large cortex cells, that can often become colourless, in contrast to the light green spine-cells. Further, the young shoots can be partially decorticated (Langangen Citation1993). The species is also characterized by the lack of a calcification process in the oospores to form gyrogonites (Soulié-Märsche and García Citation2015).
A few European studies have described the timing of the charophyte reproduction and their environmental cues (Calero, Colom, and Rodrigo Citation2015; Calero et al. Citation2017; Auderset Joye and Boissezon, Citation2017; Boissezon, Auderset Joye, and Garcia Citation2017, this Issue; Calero, Morellato, and Rodrigo Citationforthcoming), but none of them focused on C. canescens. Regarding reproductive processes, most studies describe the dimensions and morphology of the oogonia and the oospores of the species (Holzhausen et al. Citation2015; Langangen Citation1979; Mann and Nambudiri Citation2005), since the size of fructifications and some of the microscopic features have been traditionally used as taxonomic criteria (Cirujano et al. Citation2008; Krause Citation1997; Urbaniak and Gąbka Citation2015). However, there is insufficient information concerning changes in gametangia dimensions – length and width – throughout the development process in charophytes (Kwiatkowska, Pala, and Maszewski Citation1997). Further, little is known about the environmental conditions in which the fructifications are formed (Casanova Citation1997), even when salinity, light or temperature seem to affect their size (Holzhausen et al. Citation2015; Sanjuan et al. Citation2016).
C. canescens is regarded as a heliophilous species, as it can tolerate high light conditions (Küster, Schaible, and Schubert Citation2004) and prefers to live in shallow conditions below 1 m in depth (Blindow and Schubert Citation2004). The species is also considered a halophyte (Grillas, Wijck, and Bonis Citation1993), as it grows in brackish water, in salinity levels between 1.5 and 21 g/L (Blindow and Schubert Citation2004). It can even resist extreme ion anomalies (Schubert et al. Citation2017). The species tolerates from oligotrophic to highly eutrophic waters, without a clear preference for particular nutrient conditions (Blindow and Schubert Citation2004). The habitats of sexually and parthenogenetically propagating populations of C. canescens do not appear to differ (Schaible and Schubert Citation2008). However, the two distinct reproductive strategies may imply a different allocation of resources for the vegetative and the reproductive processes (as described by Dorken and Barrett [Citation2004] for monoecious and dioecious populations of an aquatic plant), so that sexual and parthenogenetic shoots might present different phenological patterns. New insights into the life cycle of the parthenogenetic populations, particularly their reproductive component, may be valuable to unravel their differences from sexual populations.
In this context, throughout 2015, we monitored the life cycle of a parthenogenetic population growing in a reconstructed shallow pond filled with brackish water and influenced by the Mediterranean Sea. It is the only described population of Chara canescens within the humid dune slacks of the Albufera de Valencia Natural Park (Spain). In this study, we extend the knowledge of the ecology of C. canescens, particularly its reproductive processes. We characterize the habitat and describe the population dynamics and the life cycle through qualitative observations of the emergence/establishment of the new shoots and quantitative data on the presence/size of the oogonia and the oospores. Our aim is to analyse whether: (i) a C. canescens population under a Mediterranean climate exhibits an annual life cycle, (ii) the size of gametangia and their position within the shoot can be used to describe the development process of the individuals of C. canescens and (iii) the dimensions of the oogonia and the oospores from the studied population are different to other populations of the same species.
Material and methods
The characterization of the study area
The studied population lived in a permanent shallow pond with sea influence, located within the dune ridges of the Albufera de València Natural Park on the East coast of Spain (39°19’40.3”N, 0°18’10.6”W). This area, named Devesa del Saler, is a 14-km long, 1-km wide sand bar that separates the Albufera de València coastal lagoon from the Mediterranean Sea (Figure A). Before an urbanization plan destroyed the dunes in the 1970s, they held several humid dune slacks with different hydro-periods and variable salinities. The seasonal rainfall and drought typical of the Mediterranean climate, as well as the bathymetry and distance to the sea, determined the water dynamics of these ecosystems. During the 2000s, the area was restored and several temporary and permanent ponds were created to mimic the former humid dune slacks (Olmo et al. Citation2016). Currently, dense populations of emergent (mainly Typha sp. and Phragmites sp.) and submerged macrophytes, including several charophyte species, inhabit these created ponds (Calero, Colom, and Rodrigo Citation2015; Calero, Morellato, and Rodrigo Citationforthcoming; Calero et al. Citation2017). The species C. canescens was previously described growing in the Devesa (Rodrigo and Alonso-Guillén Citation2016), although no exact location was provided. We found the population described in this study in November 2014; it formed a monospecific meadow in one of the restored interdunal ponds, coexisting with permanent populations of Chara hispida L. and Stuckenia pectinata (L.) Börner (Potamogeton pectinatus L.). For an accurate determination and further observations, some shoots of C. canescens were taken to our laboratory, planted in small pots and maintained in a culture room with de-chlorinated tap water, at a constant temperature (19 °C) and in artificial light (see detailed conditions in Rojo et al. Citation2017).
Figure 1. (A) The location and aerial photograph of the interdunal pond studied; taken by Matthieu Lasalle in 2015. (B) Basic features of water from the pond. TP: Total phosphorus; TN: Total nitrogen.
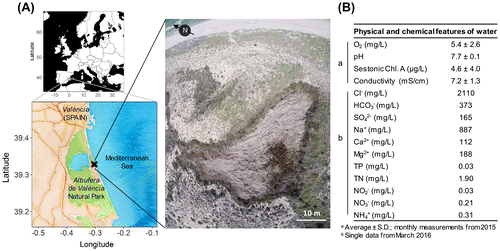
In 2015, we installed a pole with a centimetre scale to monitor the water level variations of the pond and to relate them to the local rainfall (data from the closest pluviometric station, Racó de l’Olla, Servici Devesa-Albufera, Ajuntament de València). A probe with a data logger (Onset HOBO U20–001-0, Bourne, Massachusetts, USA) was set up near the pond to continuously record the air temperature every half an hour. Some physical and chemical variables of the water (dissolved oxygen, pH and conductivity/salinity) were monitored monthly with portable field equipment throughout that year. The water samples were taken monthly to determine the sestonic chlorophyll a, as described in Calero, Colom, and Rodrigo (Citation2015). The solute composition of the water was measured by Laboratorios Tecnológicos de Levante and the nutrient concentrations by the Institute of Aquatic Engineering and Environment (UPV, Spain), using standard methods. More information about the pond inhabited by C. canescens can be found in Calero, Morellato, and Rodrigo Citationforthcoming (this pond is referred to as HSP).
The collection and analysis of charophytes
The population of C. canescens was monitored monthly from January to December 2015, with a greater frequency – biweekly – during the warmest months of the year. We focused our study on a 5-m long, 2-m wide reed-free corridor, located near the shore of the south-west side of the pond. The shallow (approximate depth 50 cm) and transparent waters allowed us to observe the sandy bottom directly in order to detect the emergence of new shoots, the growth of the established ones and, hence, to qualitatively describe the annual growth dynamic of the species. Moreover, the fresh material was collected by hand and transported to our laboratory in plastic bags, within a portable cooler. The organic matter attached to the shoots was removed with tap water to allow the detection of the reproductive organs in all the whorls, from the apex to the bottom part of the main axis, including also those in the side-branches. A total of 562 shoots (33 ± 14 shoots, mean ± SD by sampling date), were observed with a graduated ocular micrometre with maximum magnifications of 67.5× (Olympus SZ61 binocular loupe, Shinjuku, Tokyo, Japan) to detect and measure the fructifications along the whorls. When the oogonia or the oospores were present, we selected five shoots on each sampling date to measure the length (also called the longest polar axis, LPA) and width (also called the largest equatorial diameter, LED) of three fructifications per fertile whorl. The isopolarity index (ISI = LPA/LED×100) was then calculated. For the oogonia, coronula height was also measured. For a better description of the development and growth of the oogonia and the oospores, the position within the shoot (number of whorl from the apex) for each measured fructification was recorded. All the gametangia within the same whorl show a similar developmental stage, but all the sexual developmental phases can be observed within a same individual shoot. Since the charophytes develop from the apex downward, each whorl represents a different time in their development (Domańska, Godlewski, and Kobylińska Citation2013).
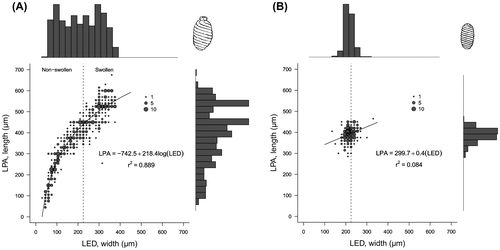
For a more detailed description of the reproductive component of the charophyte life cycle, and based on Bonis et al. (Citation1993), we defined two types of oogonia: “non-swollen”, equivalent to unripe oogonia, and “swollen”, as ripe oogonia. The relationship between both the length and the width measurements was fitted to a logarithmic model, with a first period of rapid increase for LPA values and slow for LED, followed by a slow growth period for LPA and rapid for LED. We mathematically identified the size threshold at which the length and the width of oogonia increased equally in the developmental process, so at higher values, the oogonia started to swell (see further details about the methodological approach in Calero and Rodrigo Citationforthcoming). For this population of C. canescens, we designated the oogonia as swollen when their width (LED) was equal to or above 225 μm.
The differences in the means of the oospore size variables (LPA, LED and ISI) depending on their position within the shoot were tested by means of an ANOVA test with post hoc analysis (Tukey) when n > 15.
Results
The habitat features
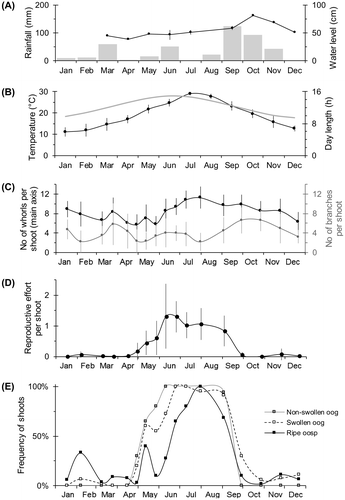
The studied population lived in a pond with oligotrophic, slightly alkaline and brackish waters (Figure B). Salinity was 3.9 ± 0.8 g/L (mean ± SD), mainly due to high concentrations of chloride and sodium. The water level in the pond remained constant throughout the year, except for a sharp increase of up to 30 cm after the autumn rainfall peak (Figure A). The daily mean temperature in the surroundings was between 5.0 °C and 30.6 °C during 2015, with values above 15.0 °C for 248 days of the year. The highest temperatures occurred in July (Figure B). At the latitude of the pond, the day length varied throughout the year from 9 to 15 h of light, with the maximum hours per day in June.
Figure 2. (A) The accumulated rainfall (bars) and the water level in the pond per month (line, mean ± SD when more than one value available). (B) The daily air temperature per month (black line, mean ± SD) at the surface of the pond, and the duration of the day (grey line) in Valencia, Spain. (C) The number of whorls of the main axis (black line) and the number of primary branches per shoot throughout the year (grey line) (mean ± SD by sampling date). (D) The reproductive effort per shoot (mean ± SD by sampling date), expressed as the number of fertile whorls per shoot (including lateral branches) divided by the number of whorls of the main axis. The higher values correspond to a higher proportion of fertile whorls per shoot, but also to a more lateral branching (presence of fertile multi-axis individuals). (E) The relative frequency of the shoots with each considered phenophase (non-swollen oogonia, swollen oogonia, ripe oospores) throughout the year, expressed as a percentage.
The population dynamics. Timing of the production of the oogonia and the oospores
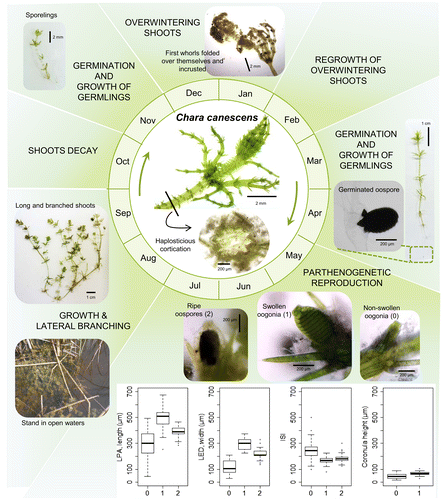
In November 2014, we detected some small individuals (a few centimetres long) that overwintered among the stems of the emergent vegetation. These shoots of C. canescens presented a dark green and greyish colour, and the branchlets of the first whorls (next to the apex part) were sometimes folded over themselves and very encrusted. Some of the shoots still bore the oospores produced during the previous reproductive period. In February 2015, these shoots started to regrow and showed small, light green sprouts, frequently partially decorticated. By March, some sporelings appeared from the germinating oospores (Figure ). From that time, C. canescens grew fast, colonized more open waters and established irregular patches along the sandy bottom of the pond. Therefore, in April we were able to find individuals with different architectures: mostly small, light green, single-axis individuals, but also some large, multi-axis individuals with basal older parts and long, new branches. As the season progressed, these single-axis individuals grew and formed more complex structures with new and longer branches (see Figure C). During the summer, C. canescens had formed quite a homogeneous stand with a healthy general appearance. The largest individuals reached 30 cm in length and presented a high number of lateral branches. After the production and the maturation of the oogonia and the oospores, the population declined. We detected some germinating oospores during the autumn. Again, by the end of the year, only some fragmented individuals remained sheltered around the submerged parts of the emergent vegetation.
Figure 3. The life cycle of a parthenogenetic population of Chara canescens in the interdunal Mediterranean pond studied. The different events are distributed throughout the months of the year in a circular diagram. At the bottom, some boxplots show the distributions of the dimensions data measured for the fructifications (LPA: longest polar axis, including the coronula in oogonia; LED: largest equatorial diameter; ISI: isopolarity index; 0: non-swollen oogonia; 1: swollen oogonia; 2: ripe oospores).
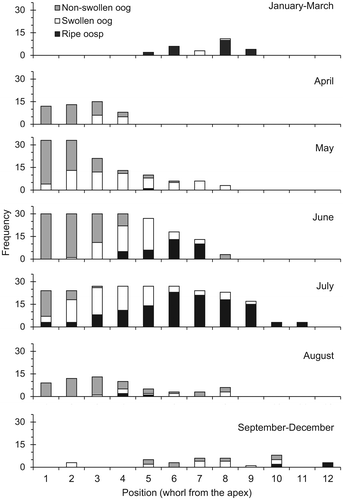
We detected only female shoots of C. canescens throughout the studied period, mainly exhibiting the oogonia and the oospores during spring and summer. The first fructifications were found in late April, when the shoots just presented a low proportion of fertile whorls (Figure D). C. canescens produced the non-swollen oogonia from the apex, simultaneously with the branchlets of its whorls, so these fructifications were mainly found in the first three whorls of the shoots (Figure ). When the first fructifications were detected, 30% of the individuals presented at least one non-swollen oogonium and 20% had at least one swollen oogonium (Figure E). As the spring season advanced, the shoots grew and most oogonia enlarged and swelled. The swollen oogonia appeared between the second and fifth whorls of the shoots, mainly between May and July (Figure ). Throughout these months, the reproductive effort per shoot increased (Figure D) as well as the frequency of shoots with all kinds of fructifications (Figure E). By 10 June, all the sampled shoots had non-swollen oogonia, and 15 days later all of them had swollen ones. Regarding the oospores, the first ones appeared less than 15 days after the emergence of the first oogonium. On some occasions, we found shoots already bearing ripe oospores when they still retained the oospore from which they had germinated. Most oospores ripened throughout June and July, being mostly present between the fifth and the ninth whorls of the shoots (Figure ). The oospore maturation peak (also 100% of individuals) happened at the end of July (Figure E), when we found up to 28–36 oospores per fertile whorl. The reproductive effort per shoot remained high until the end of September (Figure D), when the frequency of fertile individuals declined (Figure E) and no new fructifications appeared.
Figure 4. The distribution of the frequencies of all the measured oogonia and oospores in the different months of the year, according to their position within the shoot (number of whorl from the apex).
Under the stable conditions inside the culture room in our laboratory, the growth and the survival of the planted shoots was constant throughout the year, with no shoot decay. All individuals produced oospores, without seasonality and without the presence of antheridia.
The oogonium and the oospore dimensions
The length (LPA) and the width (LED) of the oogonia from C. canescens exhibited a statistically significant logarithmic relationship (Figure A), with a first period of non-swollen oogonia, followed by a swelling process that started at the defined size threshold. The non-swollen oogonia were the smallest fructifications, being 288 ± 107 μm long × 123 ± 52 μm wide (mean ± SD), and having an elongated form (higher ISI values: 240 ± 55). The wider oogonia were considered as swollen, being 497 ± 67 μm long × 296 ± 44 μm wide, with a shape and an ISI (170 ± 20) close to the value of the oospores. The ripe oospores from the studied population of C. canescens were black, small, with an ovoid shape. Their length and width showed a significant linear relationship (Figure B), with the data distributed around the size threshold for the swollen oogonia. The mean size of the oospores (±SD) was 392 ± 34 μm long × 219 ± 24 μm wide, with an ISI of 181 ± 22. Some differences between the size variables were found depending on the oospore position within the shoot (p < 0.05), although not all the positions could be tested (only whorls 4–9, when n > 15) and no clear distinctions between the groups were found (data not shown). Figure shows some photographs of the different fructifications and the data distributions of their dimensions.
Figure 5. (A) The size relationship of the oogonia and (B) the oospores from the studied population of Chara canescens, including the respective logarithmic and linear relationships (p < 0.001) between their two main biometric characteristics (LED: largest equatorial diameter; LPA: longest polar axis). As the data are overlapping, the point size is proportional to the frequency of the oogonia/oospores in each case. The histograms of the frequencies for the two variables are also shown. The dashed lines show the LED value that was considered as the threshold for the swollen oogonia.
Discussion
An annual life cycle?
Chara canescens has been described as having a monocarpic annual cycle: the shoots appear from germinating oospores in spring, reproduce only once by means of the oospores and finally die after the ripening of the fructifications (Schaible et al. Citation2012). Most individuals from the studied population, indeed, behaved this way. However, some shoots in this shallow pond on the Mediterranean coast appeared in autumn from germinating oospores (also described in the French Mediterranean region by Mouronval et al. [Citation2015]), and some were able to overwinter and survive after reproduction. Thus, this population could be split into at least two types of shoots with different life history traits. Given that during most part of the year (from May to November) all shoots were multi-axis individuals with long lateral branches, it was difficult to separate their phenological characteristics.
All the shoots from the studied population were female and reproduced parthenogenetically, like most of the populations in Europe (Schaible, Bergmann, and Schubert Citation2011). The parthenogenetic reproduction lasted five months, from April to August, in agreement with the previous observations of C. canescens (Blindow and Schubert Citation2004; Bonis et al. Citation1993). The first ripe oospores appeared only 15 days after the first non-swollen oogonia. Throughout the population, however, the lag between the oogonia formation peak and the oospores maturation peak was longer, possibly because of the above-described presence of the two types of shoots. Charophytes require a minimum time to reproduce after germination (Guha Citation1996), and even when C. canescens completes its life cycle rapidly (as seen in this population and pointed out by Blindow and Schubert [Citation2004]) some overwintering individuals developed faster in response to the environmental drivers than the shoots from germinated oospores. Either way, all the shoots produced a large number of oospores in both the main axis and the lateral branches (if present), investing a great reproductive effort.
At the beginning of the growing season, two types of plant architectures were present: single-axis shoots for those individuals that germinated from an oospore and lived for less than a year, and multi-axis shoots for those individuals that sprouted from overwintering shoots from the previous year. These two types of architecture are common in natural populations of other species of the genera, and have been related to the habitat and the capacity for clonal regeneration (Bociąg and Rekowska Citation2012). Species from shallow and likely disturbed habitats, such as C. canescens, may invest less in vegetative propagation, presenting more single-axis individuals, but more in the production of a large number of oospores. Casanova and Brock (Citation1999) described how a monoecious annual charophyte initiated its side branches only after the sexual reproduction took place, and how this strategy could lead to an early production of oospores by self-fertilization and a rapid replenishment of the propagules bank before any disturbances disrupted the process. This growth dynamic agrees with our observations in the present study of C. canescens, in spite of it being a dioecious species, but thanks to its parthenogenetic character. C. canescens is regarded as an opportunist species with low competitive capacity. When the Mediterranean ecosystems are refilled in autumn, after the summer droughts, the quick germination, growth and parthenogenetic reproduction favours the species over other submerged macrophytes, inhabiting the brackish environments (Mouronval et al. Citation2015).
The phenomenon of the overwintering has also been reported by Blindow and Schubert (Citation2004) and Mouronval et al. (Citation2015) in C. canescens populations growing at the bottom of deep ecosystems, where the environmental conditions remain constant throughout the year. We also observed that the shoots growing under constant conditions inside a culture room lost their annual character. Although shallow waters are strongly affected by the air temperature, the studied interdunal pond was located in a temperate region characterized by warm winters. Mild temperatures during the autumn and winter may allow some individuals of the species to germinate and survive when sheltered by the emergent vegetation. Since different parthenogenetic populations of C. canescens can be locally adapted to their habitats of origin (Schaible et al. Citation2012), our case study can be an example of how the environmental conditions of the Mediterranean area might lead to different trait variations and adaptations compared to northern latitudes (Thompson Citation2005).
The survival and the life cycle of C. canescens seem to be driven strongly by the environmental cues. In this population, the peak of non-swollen oogonia coincided with a high increase in the temperature and the maximum photoperiod, while the peak of ripe oospores concurred with the maximum temperatures of the year. Among the environmental factors, the light and the temperature are the main cues for the sexual reproduction of charophytes (Calero, Colom, and Rodrigo Citation2015; Calero et al. Citation2017; Calero, Morellato, and Rodrigo Citationforthcoming; Boissezon, Auderset Joye, and Garcia Citation2017, this Issue). These factors can act synergistically to trigger the production of the gametangia and oospores. We are now working with more data from this population to compare the effect of the environmental differences from year to year on the life history traits of C. canescens.
The size of fructifications: less is more
The oogonia from C. canescens grew in both width and length throughout the developmental process, according to a logarithmic equation typical from allometric relationships (Niklas Citation2004). Within this pattern, an inflexion point identified two types of oogonia, based on their width/length relation (Bonis et al. Citation1993). This distinction was useful to describe the growth of the oogonia within the shoots of C. canescens. The smaller and non-swollen oogonia mostly appeared along the first whorls – next to the apex part – while the larger and swollen oogonia were found in the lowest positions, as the shoots grew. The oogonia started to swell when the oospores began to form inside them. The swollen oogonia and their inner unripe oospores grew simultaneously, until the oospores became black and ripe, reaching the size described as the threshold for the swollen oogonia. Frequently, the oospores from the charophytes appeared “hidden” inside the oogonia due to the persistence of the integument and the coronula (Soulié-Märsche and García Citation2015). Charophytologists have traditionally included quantitative data about the specific morphology of both the oogonia and the oospores, and several studies attempted to fit equations to characterize the different species (Holzhausen et al. Citation2015; Sanjuan et al. Citation2016). However, no information about the development of the fructifications (the growth dynamics or the ecological conditions) are included, not even in specialized literature. For the sake of data comparability, we propose to include this information at least for those species whose fructification dimensions change notably with the development and between the populations.
In this population of C. canescens, both the oogonia and the oospores were really small and, in fact, out of the ranges described in the literature (Table ; more authors quoted in Holzhausen et al. [Citation2015]). These data show the great variability found within a same charophyte species, even when it reproduces asexually (by parthenogenesis). The oospores were clearly viable and enabled the establishment and persistence of the population year after year. Some studies have suggested a role of the habitat in the development of the fructifications and the variation of the oospores (Casanova Citation1997). In the paleontological records, small gyrogonites (some of them in the range described for C. canescens oospores from this study) have been found in extremely shallow environments with high temperature and light availability (Vicente et al. Citation2016). Sanjuan et al. (Citation2016) described how Chara vulgaris produced smaller gyrogonites under experimental conditions with higher temperature and light intensity. Thus, some authors have pointed out that the fructifications from both fossil and extant charophytes growing in shallow temporary ponds may be subjected to natural selection for size (Casanova Citation1997) in a long history of adaptation to these unstable habitats. The charophyte species inhabiting such ecosystems would present opportunistic strategies, producing a high number of small gyrogonites in short life cycles (a greater number, but smaller in size due to ecological trade-offs [Stearns Citation1989]). In the extant populations, the same charophyte species shows a shorter life cycle and a higher production of oospores when growing under shallower conditions (Asaeda, Rajapakse, and Sanderson Citation2007). In this context, the presence of small oospores in this population could be reasonable. Even though the studied population currently inhabits a permanent pond, the area is subject to a Mediterranean climate and was formerly occupied by humid dune slacks, most of them temporary. Thus, all the selective pressures from this habitat would have led to the main life history traits described for this population of C. canescens: the species is able to produce a high number of small oospores – smaller than any other described population – and in a short life time.
Table 1. The size ranges described in the literature for the swollen oogonia and the oospores from Chara canescens. LPA: longest polar axis, including the coronula in oogonia (marked with * if the coronula was excluded); LED: largest equatorial diameter; ISI: isopolarity index. 1: Groves and Bullock-Webster (Citation1924); 2: Krause (Citation1997); 3: Mann and Nambudiri (Citation2005); 4: Cirujano et al. (Citation2008); 5: Urbaniak and Gąbka (Citation2015); 6: Calero and Rodrigo (this study).
Concluding remarks
This study describes two coexisting life cycles within a parthenogenetic population of C. canescens inhabiting a Mediterranean interdunal pond. On one hand, we found annual individuals with a short life cycle and only one reproduction event in their lives, in accordance with the previously described cycle and the opportunistic character of the species. On the other hand, as the phenology of C. canescens seems to be particularly determined by a seasonal environment, mild winter temperatures in the pond allowed some shoots to overwinter and reproduce more than once. This finding confirmed that life history traits vary in Mediterranean climates, compared to northern Europe, as an adaptation to their different conditions (Thompson Citation2005). Regardless of the life cycle, the species produced a high number of small fructifications, agreeing with the expected strategy for a species from shallow, temporary habitats. This study has contributed to the ecological knowledge of C. canescens and will be helpful in the design of conservation and/or restoration plans involving charophytes.
C. canescens is a particularly interesting species, as its parthenogenetic character might help us to understand the underlying mechanisms for plant sex differentiation (Braun et al. Citation2007). Knowing more about its phenology and the life cycle may help us to better understand the differences between the sexual and the parthenogenetic populations of the species, as well as the overall process of the gametangia development in charophytes. Even though there is published information about the life cycle of C. canescens in other parts of Europe, a lot of useful data are scattered and not available in English (some of them summarized by Blindow and Schubert [Citation2004]). Thus, there are still some challenges to overcome when trying to unravel the life cycle of this and other species of charophytes. More studies concerning their reproductive aspects – including their development, phenology and ecological requirements for oospore formation – will improve our knowledge of the ecology of the species.
Funding
This work was supported by the Spanish Ministry of Education, Culture and Sport under Grant FPU13/02254 to SC.
Notes on contributors
Sara Calero is an ecologist and researcher in training at the University of Valencia. She is currently working on the life cycle of different submerged macrophytes to unravel the potential effects of climate change on aquatic ecosystems. Contribution: collected and analysed data, prepared and wrote the manuscript.
Maria A. Rodrigo is an aquatic ecologist and full-time professor at the University of Valencia. For the last 10 years, she has been working with charophytes from Mediterranean aquatic ecosystems, particularly concerning the effects of global change on them. Contribution: planned research work, collected data, reviewed the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
Local and regional authorities facilitated permission to study the area (Oficina Tècnica Devesa-Albufera, Ajuntament de València; Servei de Parcs Naturals, Generalitat Valenciana). We are thankful to Andrzej Pukacz, who discovered this population with us, and to Anna Escolano-Moltó and other collaborators from the Integrative Ecology Lab, who kindly helped with the field work. Daniel Sheerin (Online English S.C.), a native English teacher who specializes in scientific English, and Mary J. Beilby reviewed and corrected the language in the manuscript.
References
- Asaeda, T., L. Rajapakse, and B. Sanderson. 2007. “Morphological and Reproductive Acclimations to Growth of Two Charophyte Species in Shallow and Deep Water.” Aquatic Botany 86: 393–401.10.1016/j.aquabot.2007.01.010
- Auderset Joye, D., and A. Boissezon. 2017. “New Insights Into the Ecology and Phenology of Two Nitella: N. opaca (Bruzelius) C. Agardh and N. gracilis (Sm.) C. Agardh.” Botany Letters. (this issue). doi: 10.1080/23818107.2017.1365259.
- Blindow, I., and H. Schubert. 2004. “Chapter 4.5. Chara canescens Desv. & Loisel. In Loisel. 1810.” In Charophytes of the Baltic Sea, edited by H. Schubert and I. Blindow, 70–81. Ruggell: A.R.G. Gantner Verlag Kommanditgesellschaft.
- Bociąg, K., and E. Rekowska. 2012. “Are Stoneworts (Characeae) Clonal Plants?” Aquatic Botany 100: 25–34.10.1016/j.aquabot.2012.03.007
- Boissezon, A., D. Auderset Joye, and T. Garcia. 2017. “Temporal and Spatial Changes in Population Structure of the Freshwater Macroalgae Nitellopsis obtusa (Desv.) J. Groves.” Botany Letters (this issue). doi: 10.1080/23818107.2017.1356239.
- Bonis, A., P. Grillas, C. Wijck, and J. Lepart. 1993. “The Effect of Salinity on the Reproduction of Coastal Submerged Macrophytes in Experimental Communities.” Journal of Vegetation Science 4: 461–468.10.2307/3236073
- Braun, M., I. Foissner, H. Löhring, H. Schubert, and G. Thiel. 2007. “Characean Algae: Still a Valid Model System to Examine Fundamental Principles in Plants.” Progress in Botany 68: 193–220.10.1007/978-3-540-36832-8
- Brock, M. A., and M. T. Casanova. 1991. “Plant Survival in Temporary Waters: a Comparison of Charophytes and Angiosperms.” Verhandlungen Internationale Vereiningung für Theroretische und Angewandte Limnologie 24: 2668–2672.
- Calero, S., W. Colom, and M. A. Rodrigo. 2015. “The Phenology of Wetland Submerged Macrophytes Related to Environmental Factors.” Limnetica 34: 425–438.
- Calero, S., D. Auderset Joye, A. Rey-Boissezon, and M. A. Rodrigo. 2017. “Time and Heat for Sexual Reproduction: Comparing the Phenology of Chara hispida of Two Populations at Different Latitudes.” Aquatic Botany 136: 71–81.10.1016/j.aquabot.2016.09.011
- Calero, S., L. P. C. Morellato, and M. A. Rodrigo. forthcoming. “Persistence of Submerged Macrophytes in a Drying World: Unravelling the Timing and the Environmental Drivers for the Production of Drought-Resistant Propagules.” Aquatic Conservation: Marine and Freshwater Ecosystems.
- Calero, S., and M. A. Rodrigo. forthcoming. A Quantitative Method to Analyse the Sexual Development of Charophytes: a Baseline for Phenological Studies.
- Casanova, M. T. 1994. “Vegetative and Reproductive Responses of Charophytes to Water-Level Fluctuations in Permanent and Temporary Wetlands in Australia.” Marine and Freshwater Research 45: 1409–1419.10.1071/MF9941409
- Casanova, M. T. 1997. “Oospore Variation in Three Species of Chara (Charales, Chlorophyta).” Phycologia 36: 274–280.10.2216/i0031-8884-36-4-274.1
- Casanova, M. T., and M. A. Brock. 1999. “Life Histories of Charophytes from Permanent and Temporary Wetlands in Eastern Australia.” Australian Journal of Botany 47: 383–397.10.1071/BT97086
- Cirujano, S., J. Cambra, P. Sánchez Castillo, A. Meco, and N. Flor Arnau. 2008. Flora Ibérica. Algas Continentales. Carófitos (Characeae). [Flora Iberica. Continental Algae. Charophytes (Characeae)]. Madrid: Real Jardín Botánico, CSIC-Universidad de Barcelona.
- Domańska, A., M. Godlewski, and A. Kobylińska. 2013. “Effect of cAMP Level on Thallus Growth and Gametangia Development in Macroalga Chara vulgaris (Charales, Charophyta).” Acta Biologica Cracoviensia Series Botanica 55: 90–98.
- Dorken, M. E., and S. C. Barrett. 2004. “Phenotypic Plasticity of Vegetative and Reproductive Traits in Monoecious and Dioecious Populations of Sagittaria latifolia (Alismataceae): A Clonal Aquatic Plant.” Journal of Ecology 92: 32–44.10.1111/jec.2004.92.issue-1
- Forrest, J., and A. J. Miller-Rushing. 2010. “Toward A Synthetic Understanding of the Role of Phenology in Ecology and Evolution.” Philosophical Transactions of the Royal Society B 365: 3101–3112.10.1098/rstb.2010.0145
- Grillas, P., C. Wijck, and A. Bonis. 1993. “The Effect of Salinity on the Dominance-Diversity Relations of Experimental Coastal Macrophyte Communities.” Journal of Vegetation Science 4: 453–460.10.2307/3236072
- Groves, J., and G. R. Bullock-Webster. 1924. The British Charophyta, Volume II. Chareae. London: The Ray Society.
- Guha, P. 1996. “Phenology of Sexual Reproduction of Chara fibrosa and Chara zeylanica.” Thai Journal of Agricultural Science 29: 301–309.
- Holzhausen, A., P. Nowak, C. Niedrig, M. Feike, and H. Schubert. 2015. “Morphometry of Chara aspera, C. canescens, C. baltica var. baltica, C. baltica var. liljebladii and C. intermedia oospores: Local Variation Versus Taxonomic Differences.” Aquatic Botany 120: 60–66.10.1016/j.aquabot.2014.07.001
- IPCC. 2014. “Climate Change 2014: Synthesis Report.” In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, edited by Core Writing Team, R. K. Pachauri, and L. A. Meyer, 151pp. Geneva: IPCC.
- Krause, W. 1997. “Charales (Charophyceae).” In Sübwasserflora von Mitteleuropa [Pure Water Flora of Middle Europa], edited by H. Ettl, G. Gärtner, H. Heynig, and D. Mollenhauer, Vol. 18, 202. Stuttgart: Fischer.
- Küster, A., R. Schaible, and H. Schubert. 2004. “Light Acclimation of Photosynthesis in Three Charophyte Species.” Aquatic Botany 79: 111–124.10.1016/j.aquabot.2004.01.010
- Kwiatkowska, M., A. Pala, and J. Maszewski. 1997. “Size-Variation in the Antheridia and Oogonia of Chara vulgaris Under Different Experimental Conditions.” Acta Societatis Botanicorum Poloniae 66: 29–32.
- Langangen, A. 1979. “Chara canescens Reported from Spitsbergen.” Phycologia 18: 436–437.10.2216/i0031-8884-18-4-436.1
- Langangen, A. 1993. “Some Morphological and Ecological Observations on Chara canescens (Charophyte).” Cryptogamie. Algologie 14: 215–220.
- Mann, H., and E. M. V. Nambudiri. 2005. “Charophytes of Insular Newfoundland II: Chara evoluta and Chara canescens.” The Canadian Field-Naturalist 119: 26–37.10.22621/cfn.v119i1.77
- Mouronval, J. B., S. Baudouin, N. Borel, I. Soulié-Märsche, M. Klesczewski, and P. Grillas. 2015. Guide des Characées de France Méditerranéenne [Guide of Characeae of Mediterranean France]. Paris: Office National de la Chasse et de la Faune Sauvage.
- Niklas, K. J. 2004. “Plant Allometry: Is There A Grand Unifying Theory?” Biological Reviews 79: 871–889.10.1017/S1464793104006499
- Olmo, C., X. Armengol, M. Antón-Pardo, and R. Ortells. 2016. “The Environmental and Zooplankton Community Changes in Restored Ponds Over 4 Years.” Journal of Plankton Research 38: 490–501.10.1093/plankt/fbw021
- Rodrigo, M. A., and J. L. Alonso-Guillén. 2013. “Assessing the Potential of Albufera de València Lagoon Sediments for the Restoration of Charophyte Meadows.” Ecological Engineering 60: 445–452.10.1016/j.ecoleng.2013.09.041
- Rodrigo, M. A., and J. L. Alonso-Guillén. 2016. “The Charophyte Flora in a Ramsar Mediterranean Wetland (Albufera de València Natural Park, Spain) During the Period 2007-2010.” Botanica Serbica 40: 205–215.
- Rodrigo, M. A., J. L. Alonso-Guillén, and I. Soulié-Märsche. 2010. “Reconstruction of the Former Charophyte Community Out of the Fructifications Identified in Albufera de València lagoon Sediments.” Aquatic Botany 92: 14–22.10.1016/j.aquabot.2009.09.002
- Rojo, C., M. Carramiñana, D. Cócera, G. P. Roberts, E. Puche, S. Calero, and M. A. Rodrigo. 2017. “Different Responses of Coexisting Chara Species to Foreseeable Mediterranean Temperature and Salinity Increases.” Aquatic Botany. 138: 53–63.
- Sanjuan, J., A. Vicente, N. Flor-Arnau, T. Monleón, J. Cambra, and C. Martín-Closas. 2016. “Effects of Light and Temperature on Chara vulgaris (Charophyceae) Gyrogonite Productivity and Polymorphism – Palaeoenvironmental Implications.” Phycologia 56: 204–212.
- Schaible, R., and H. Schubert. 2008. “The Occurrence of Sexual Chara canescens Populations (Charophyceae) is not Related to Ecophysiological Potentials with Respect to Salinity and Irradiance.” European Journal of Phycology 43: 309–316.10.1080/09670260801930348
- Schaible, R., I. Bergmann, M. Bögle, A. Schoor, and H. Schubert. 2009. “Genetic Characterisation of Sexually and Parthenogenetically Reproductive Populations of Chara canescens (Charophyceae) Using AFLP, rbc L, and SNP Markers.” Phycologia 48: 105–117.10.2216/08-58.1
- Schaible, R., I. Bergmann, and H. Schubert. 2011. “Genetic Structure of Sympatric Sexually and Parthenogenetically Reproducing Population of Chara canescens (Charophyta).” ISRN Ecology 2011. Article ID 501838, 13 pages. doi:10.5402/2011/501838.
- Schaible, R., A. Gerloff-Elias, F. Colchero, and H. Schubert. 2012. “Two Parthenogenetic Populations of Chara canescens Differ in their Capacity to Acclimate to Irradiance and Salinity.” Oecologia 168: 343–353.10.1007/s00442-011-2113-y
- Schubert, H., I. Telesh, M. Nikinmaa, and S. Skarlato. 2017. “Physiological Adaptations.” In Biological Oceanography of the Baltic Sea, edited by P. Snoeijs-Leijonmalm, H. Schubert, and T. Radziejewska, 255–278. Dordrecht: Springer.10.1007/978-94-007-0668-2
- Soulié-Märsche, I., and A. García. 2015. “Gyrogonites and Oospores, Complementary Viewpoints to Improve the Study of the Charophytes (Charales).” Aquatic Botany 120: 7–17.10.1016/j.aquabot.2014.06.003
- Stearns, S. C. 1989. “Trade-Offs in Life-History Evolution.” Functional Ecology 3: 259–268.10.2307/2389364
- Thompson, J. D. 2005. Plant Evolution in the Mediterranean. New York: Oxford University Press.10.1093/acprof:oso/9780198515340.001.0001
- Urbaniak, J., and M. Gąbka. 2015. Polish Charophytes. An Illustrated Guide to Identification. Wrocław: Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu.
- Vicente, A., M. Expósito, J. Sanjuan, and C. Martín-Closas. 2016. “Small sized charophyte gyrogonites in the Maastrichtian of Coll de Nargó, Eastern Pyrenees: An adaptation to temporary floodplain ponds.” Cretaceous Research 57: 443–456.10.1016/j.cretres.2015.07.017
