Abstract
The coastal waters are important transition zones for terrestrial nutrient and pollutant runoff and the open Sea. During the last decades, eutrophication has negatively influenced macrophyte communities of the coastal ecosystems, making restoration activities inevitable. This study analysed recent macrophyte vegetation and the reproductive potential through the sediment diaspore reservoir along the German Baltic Sea coastline. Salinity was identified to be the responsible factor for shifts in the macrophyte community with most commonly found species like Stuckenia pectinata or Myriophyllum spicatum. In contrast, the oospores of small charophytes (e.g. Tolypella nidifica) clearly dominated the sediment diaspore bank. The germination rates differed in the recent vegetation composition and the diaspore bank composition with Zannichellia palustris as the dominant species. However, several species not visually detected at the respective sites were represented in the diaspore bank and germinated at a low rate (e.g. Chara contraria and Lamprothamnium papulosum). The maximal germination frequency corresponded to the sediment layer in which diaspore density was the highest (5–15 cm). In conclusion, the germinable diaspores were observed at all sites. Considering the differences between the diaspore composition, recent vegetation and germination success, we have illustrated the potential of the diaspore banks for the restoration of the macrophyte communities after at least mid-term periods of disturbance.
Introduction
The increasing anthropogenic pressure affects the coastal ecosystems of the Baltic Sea for the last century, resulting in drastic changes in the species composition and matter flux (e.g. Schernewski and Schiewer Citation2002). Macrophytes dominated the sheltered areas leeward of isles, coastal inlets and lagoons, but disappeared from their maximum growth depth, and the species composition shifted from charophytes and seagrasses towards opportunistic higher plant species (Krause-Jensen et al. Citation2008). In extreme cases, the changes of sediment parameters led to an extinction of macrophytes, while the phytoplankton drastically increased (Selig et al. Citation2007). Macrophytes are a key element of organismic biodiversity, structuring the habitats and the nutrient flows, and their disappearance influences all trophic levels (Schiewer Citation1997). The legal regulations for the sustainable management of aquatic ecosystems in Europe, such as the EU Water Framework Directive (EU-WFD), assess the current state as unacceptable (European Union Citation2000). Consequently, the appropriate measures for restoration of the respective ecosystems, including macrophytes, have to be considered (Selig et al. Citation2007).
In all cases, the nutrient input from point and diffuse sources needs to be identified (Schlungbaum and Baudler Citation1998). For the large and open ecosystems, such as the Baltic coastline, the amount of nutrients in the sediment is still not sufficiently investigated, and it may take decades until the active sediment nutrient pool is reduced, so that macrophytes can re-establish (Jeppesen et al. Citation2007). Further, standard methods for lake restoration, such as sediment removal, are not applicable for the coastal systems (Schiewer Citation2008). Another option is the re-colonization with the available macrophyte inventory, which can be the living organisms entering the system from the neighbouring undisturbed sites, the persistent diaspores of the system itself, or the diaspores imported by the water currents or other vectors. The Baltic Sea is distinguished by a steep salinity gradient along the German coastline (Lass and Matthäus Citation2008), which has been shown to be a “master factor” for the species distribution including macrophytes (e.g. Schubert et al. Citation2011; Telesh, Schubert, and Skarlato Citation2013). Consequently, the salinity ranges must be considered for the vegetative growth and successful reproduction to estimate the realization potential of the diaspores. Moreover, the diaspores of the annual species have not evolved for a long-distance dispersal, as they are adapted to seasonality and expected to persist only for a limited time to germinate after short periods of dormancy (Selig, Steinhardt, and Schubert Citation2009).
To evaluate the restoration potential of the “status quo ante”, the site-specific diaspore content and the germination success need to be investigated. The restoration might be supported by, for instance, planting or inoculation, but these could cause problems such as the disturbance of the source populations, gene pool contamination et cetera (e.g. Barett and Kohn Citation1991). Therefore, the activation of the existing realization potential always has to be prioritized (Rodrigo and Alonso-Guillén Citation2013).
A detailed investigation of the diaspore potential does not exist for the coastal ecosystems of the southern Baltic Sea. Preliminary studies (Steinhardt and Selig Citation2009) showed severe discrepancies between the recent vegetation and the diaspore composition with further investigation required. We hypothesized that within an extended range of the coastal sites, at least some single sites could reveal consistent patterns of relationships between the diaspore abundance, current vegetation and potential germination success. The search for the corresponding sites would provide a new starting point for the analysis of environmental parameters, including reference points, where the three basic biological parameters for the succession of vegetation have synergistic constellations and/or coincident efficacy. The existence of such reference sites would allow a reassessment of the extraordinary variability of the environmental factors that could potentially affect the success of colonization by the macrovegetation in coastal regions. The demonstration of the germinable diaspores of rare species, which are promising for restoration activities, plays a special role in assessing the active memory of corresponding sites.
Material and methods
Study area
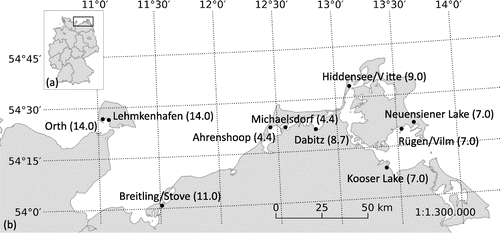
The macrophyte communities and sediments were analysed at ten sampling sites along the Baltic coastline from the islands Fehmarn to Rügen, north eastern Germany (Figure ). The study areas contained the outer shores, coastal lagoons and coastal lakes and were characterized by a high variability and intensity of various environmental factors: especially salinity and eutrophication, but also temperature, resuspension, sediment composition, hydrographical factors and exposure (Sagert, Selig, and Schubert Citation2008). Due to the variable habitat characteristics, such as the sediment structure or the turbidity of the water column, not all the analyses could be completed for each site. The detailed numbers of the survey locations are given at the beginning of each method description.
Vegetation cover
The vegetation was investigated between June and September 2014. The coverage of the submersed plants was determined by snorkelling and by defining 5 m2 quadrate areas at five sampling sites, located at the depths of 1 m and 2 m (Orth, Breitling/Stove, Dabitz, Rügen/Vilm, Ahrenshoop) and at Lehmkenhafen, Neuensiener Lake and Kooser Lake located only at depths of 1 m, because of shallow water conditions. Due to the high turbidity at Michaelsdorf and Hiddensee/Vitte, determination was not possible. The angiosperm macrophytes were determined following Rothmaler, Schubert, and Vent (Citation1994). The charophytes were identified to a species level following Krause (Citation1997). The percentage cover of each species was estimated using a modified and combined Braun-Blanquet abundance dominance scale (Table , Ellenberg Citation1992).
Table 1. Determination of the degrees of vegetation coverage in (%) after transformation in accordance with Ellenberg (Citation1992) coverage (ind.: individual).
Sediment and diaspore analyses
The sediment samples for the diaspore bank analyses were collected between May and September 2014 with a sediment corer (UWITEC, Mondsee, Austria). At the sites Orth, Breitling/Stove, Michaelsdorf and Hiddensee/Vitte the samples were collected at 1 m and 2 m depth, and at the sites Lehmkenhafen, Kooser Lake, Neuensiener Lake and Rügen/Vilm, only the samples from 1 m water depth were taken. At these locations, the 2 m depth either did not exist or exhibited an already significantly altered sediment structure (Rügen/Vilm: hard substrate). Five replicate cores (30 cm long, 5.5 cm diameter) were taken at each sampling site. The cores were cut into 5 cm layers in the laboratory and 50 ml of each layer, and each core replicate was used for the diaspore analyses and the germination experiments.
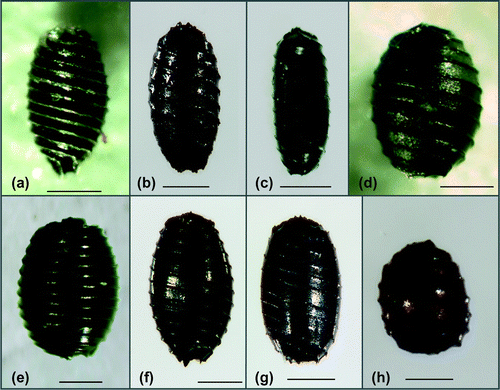
For the diaspore analyses, the sediment samples were washed through the sieves with mesh sizes of 1, 0.5 and 0.2 mm. The fractions were dried at 60°C for 12 h and inspected under a dissecting microscope (Olympus SZX2-ILLT, Olympus Inc., Tokyo, Japan). The visually intact and defective diaspores were separated. The intact oospores had an undamaged membrane that did not undergo deformation when pressed with forceps (Casanova and Brock Citation1990). Based on an average of the five replicate cores, the diaspore abundances (number per litre) were determined for each site and sediment layer. The seeds of the angiosperms (Figure ) were identified using the determination keys of Bertsch (Citation1941) and Beijernick (Citation1947); the taxonomy of all species reported here is established in the floras. The oospores were identified using basically the determination key of Vedder (Citation2004) and complemented by the literature of Krause (Citation1997). Due to the huge variability of the morphological parameters within the taxa, the applicability of the oospore determination keys especially for the brackish water material is limited. The groups identified in this study are based on the clustering developed by Vedder (Citation2004) especially for oospores of the Baltic Sea: (1) C. aspera Willdenow: oospores shorter or equal 575 μm, shiny black, ridges prominent, prolonged into conspicuous basal claws or cage (Figure (a)). (2) Chara canescens Loiseleur/C. aspera group: oospores 525–650 μm long, black, ridges low, without or only short basal claws. This group contained mainly C. canescens oospores (Figure -c, d). Additionally, intermediate oospores with features of C. canescens and C. aspera were included in this cluster (Figure (b)). Chara canescens oospores are very variable and exhibit different forms (ovoid, spindle-shaped or elongated; Krause Citation1997). Due to abrasion during sediment storage, the determination features (prominent ridges, prolonged claws or cage) of C. aspera oospores could be lost. A differentiation of both species based on the length of their oospores is not possible. (3) C. baltica (Hartman) Bruzelius/C. horrida Wahlstedt group: the oospores equal or longer than 650 μm, black, ridges mostly prominent, without claws at the apical and basal contour. This group contained the larger oospores from C. baltica and C. horrida (Figure (e)). (4) C. globularis Thuiller/C. virgata Kützing group: containing the medium-sized C. globularis and C. virgata oospores (550–750 μm) with mostly prominent ridges. The compact apical rosette permitted a clear determination of those oospores (Figure (f)). If this character was not clearly visible, the oospores of these taxa were assigned to group 2 (550–650 μm) or 3 (>650 μm). (5) Lamprothamnium papulosum (Wallroth) J. Groves the oospores with typically cylindrical outline (500–625 μm), brown to black, ridges undulated, commonly prolonged into short basal and apical points (Figure (g)). (6) Tolypella nidifica (Müller) A. Braun the oospore spherical with flattened basal pole, showing the mark of the multipartite basal plate, prominent ridges, 325–450 μm long, 275–375 μm wide, brown to black (Figure (h)).
Figure 2. Lateral view of the angiosperm seeds (scale bars = 1 mm). (a) Stuckenia pectinata (Michaelsdorf, 0–5 cm sediment depth); (b) Ruppia cirrhosa (Rügen/Vilm, 0–5 cm sediment depth); (c) Zostera marina (Rügen/Vilm, 0–5 cm sediment depth); (d) Najas marina (Michaelsdorf, 0–5 cm sediment depth); (e) Zannichellia palustris (Rügen/Vilm, 0–5 cm sediment depth).
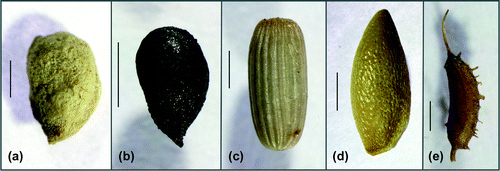
Figure 3. Lateral view of the oospores of the charophytes (scale bars = 200 µm). (a) C. aspera with prominent ridges and basal cage (Michaelsdorf, 0–5 cm sediment depth); (b) intermediate form of the C. canescens/C. aspera group without basal cage (Michaelsdorf, 5–10 cm sediment depth); (c) C. canescens, elongated form (Breitling/Stove, 0–5 cm sediment depth); (d) C. canescens, ovoid form (Orth, 0–5 cm sediment depth); (e) C. baltica/C. horrida group (Rügen/Vilm, 0–5 cm sediment depth); (f) C. globularis/C. virgata group with a apical rosette (Breitling/Stove, 0–5 cm sediment depth); (g) L. papulosum (Lehmkenhafen, 5–10 cm sediment depth); (h) T. nidifica (Breitling/Stove, 0–5 cm sediment depth).
Germination experiments
The germination experiments were performed using the sediment samples from Orth, Lehmkenhafen, Breitling/Stove, Michaelsdorf, Dabitz, Hiddensee/Vitte, Rügen/Vilm, Neuensiener Lake and Kooser Lake. Based on the results of Steinhardt and Selig (Citation2011) the germination frequency decreases drastically below the 0.4 cm sediment depth. Therefore, 50 ml of each sediment layer was incubated in glass beakers (500 ml), yielding less than 1 cm sediment depth that would provide optimal germination conditions (light). Each beaker was covered with the water from the sampling site for five months at constant temperature (15°C) and light conditions (12:12 L:D light regime, 80–90 μmol photons m−2 s−1). After 5 months, the seedlings were counted and determined at species level, using the literature previously cited. For the emerged charophytes that did not develop sufficient morphological characteristics, the species determination was carried out by genetic analyses as described below. Additionally, the individuals germinated from seeds, oospores or vegetative structures were identified; this action ended the experiment by careful removal of all seedlings.
Genetic analyses
The genetic analyses were used to confirm the results of morphologically determined charophyte oospores. The oospores from the uppermost (0–5 cm) and the lowest (25–30 cm) layer of the sediment cores from three sites (Orth, Kooser Lake, Breitling/Stove) were initially grouped along the morphological characteristics. Forty oospores from each layer (if a respective number was available) were selected for genetic analyses, ensuring that the oospores of each main morphological group were chosen. The oospores were photographed using a calibrated BH2 microscope (Olympus Inc., Tokyo, Japan), and the morphologically important parameters (length, width, number of ridges, fossa average) were measured, using the image-analysis software ImageJ (Abramoff, Magalhães, and Ram Citation2004). The individual oospore was homogenized in the innuSPEED Lysis Tubes (Analytik Jena AG, Jena, Germany) containing one zirconium bead (3 mm). The tubes were shaken vigorously at 4800 rpm for 20 s on a 3110BX mini-beadbeater (Biospec Products Inc., Bartlesville, OK, USA) and were employed directly for the preparation of genomic DNA by the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). The amplifications of the plastid markers rbcL (420 bp) and matK (1235 bp) were performed using Characeae-specific primers (Table S1). The PCR reactions were performed with a Taq PCR Master Mix (Qiagen, Hilden, Germany), and with the previously described PCR conditions for matK (Nowak, Schubert, and Schaible Citation2016) and for rbcL (Perez et al. Citation2014). The sequences were compared with the intern database of the Aquatic Ecology Department, University of Rostock, to determine the oospores at a species level. The correlation between the morphological and the genetic determinations was evaluated with a discrimination analysis in SPSS (IBM SPSS statistics, Version20) for the three main genetic groups: T. nidifica, C. hispida group and C. canescens. Because of the small number, the middle-sized oospores were not included in the analysis.
Results
Vegetation
At the 2 m water depths of Michaelsdorf and Ahrenshoop, no vegetation was observed. The high turbidity made analyses of the vegetation coverage impossible at Hiddensee/Vitte (1 m and 2 m water depth) and Michaelsdorf (1 m water depth). At the remaining sampling sites, the species composition and coverage (Table ) were quite variable. The vegetation analyses yielded a total of 10 species of submerged macrophytes: five angiosperms and five charophytes, but no species was found at all the sites. The frequently found taxa were Stuckenia pectinata (L.) Börner (five sites), Zannichellia palustris L., Ruppia cirrhosa (Petagna) Grande, C. aspera and C. canescens (four sites). Zostera marina L. was found only at the site Orth, and L. papulosum and T. nidifica were found only in Lehmkenhafen. The coverage at most sites was dominated by the angiosperms with an average of about 50%. The charophyte communities provided only 20% coverage of the vegetation, and at two stations only the charophytes dominated over the macrophyte vegetation (30% at Dabitz 1 m and 80% at Rügen/Vilm 2 m). However, the species compositions at 1 and 2 m at a sampling site were similar and differed mainly in the abundances of the observed taxa.
Table 2. Relative coverage of the macrophyte vegetation at the sampling sites (–: depth not present, x: no data).
Diaspores
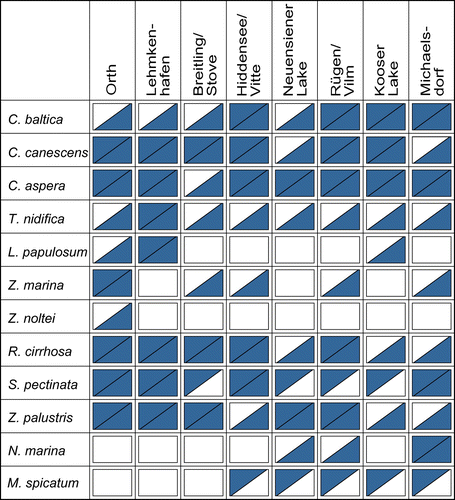
The diaspores of six angiosperms and five charophyte groups were found in the silty-sandy sediment samples. The sediment parameters were very heterogeneous and reflected mainly the degree of the exposure of the different sites. The species composition was different among the stations, but in contrast to the recent vegetation, the oospores formed around 90% of the entire diaspore bank (Table ). The oospores of L. papulosum were found only at Orth, Lehmkenhafen and Kooser Lake. The oospores of the remaining charophyte groups were present at all sites, whereas T. nidifica (50%) and the C. aspera/C. canescens group (30%) constituted the major part in the entire diaspore bank. The angiosperm diaspores were dominated by those of Z. palustris (Figure (e)), with a relative abundance of 7% for the analysed sites. The seeds of Z. palustris of the Ruppia sp. group (Figure (b)) were present at all sampling sites, the latter accounting for only 0.5% of the entire diaspore bank. The seeds of all the remaining four angiosperms formed around 1% of the diaspore bank and were observed at one (Zostera noltei Hornemann) to a maximum of five (Z. marina, Figure (c)) sites.
Table 3. Diaspore distribution calculated as an average of the five sediment cores (number per litre) collected at each sampling site (water depth; m) and given for the different sediment depth layers (cm). Numbers in parentheses denote intact diaspores (%).
Although the seed abundance was quite variable, the seeds of L. papulosum and Z. marina were more abundant at the sites with higher salinities. In contrast, the seeds of Najas marina L. or the oospores of C. aspera and C. baltica/C. horrida were found with higher abundances at the sites with lower salinities.
The differentiation between the visually intact and the defective diaspores revealed that only around 15% of the diaspore bank contained visually intact diaspores, but differences in preservation among the different taxa were observed. Within the angiosperms, the seeds of Z. noltei were only found in Orth. Even at this site, the abundances of Z. noltei seeds were very low, with all of them visually defective. In contrast, the N. marina seeds (Figure (d)) were observed at three sites, and 100% were visually intact at Rügen/Vilm. The differences within the charophytes ranged between 25% intact oospores for the C. aspera/C. canescens group and only 6% visually intact oospores for the C. baltica/C. horrida group (Table ).
The diaspores were mainly concentrated in the upper of the six different sediment layers (0–15 cm), but the intact diaspores were also found in the deeper parts from 20 to 30 cm (Table , Figure ). The proportion of the intact diaspores decreased with increasing depth of the sediment layer.
Figure 4. Average distribution of the intact and the defective diaspores (%) of the investigated sediment layers per homogenized sediment weight and summarized for the eight analyzed sites.
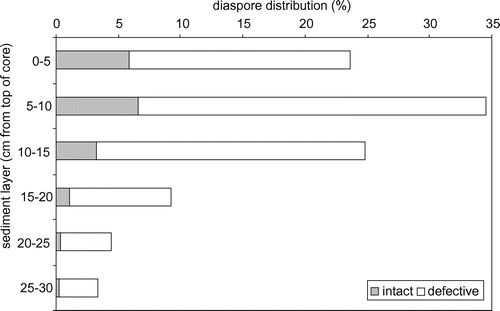
Comparing the presence and the absence of taxa in the recent vegetation and the diaspore reservoir (Figure ), the charophyte taxa, like C. canescens and C. aspera, were present at almost all sites. The same holds true for the angiosperm taxa Z. palustris or R. cirrhosa. Interestingly, while the oospores of T. nidifica were present at all analysed sites, it has grown only in Lehmkenhafen. Zostera marina plants were present in the recent vegetation only in Orth, but their seeds were found in the sediments of four other sites. Stuckenia pectinata (four sites) and Myriophyllum spicatum L. (five sites) were present in the recent vegetation, but without diaspores in the sediment.
Genetic determination
For the genetic analysis, 225 single oospores were treated, and 198 were determined genetically, with both the visually intact and the defective oospores showing positive PCR signals. The obtained sequence lengths were quite variable (Table ), with an average length of the obtained sequences between 400 and 600 bp.
Table 4. Number of base pairs (bp) obtained for rbcL and matK sequences during genetic analyses of single oospores.
The plastid rbcL and the matK sequences revealed six different genetic taxa. Interestingly, none of the oospores from the morphological C. aspera/C. canescens group (N = 61) were identified as C. aspera. A main cluster belonged to the diplostichous C. hispida group. Due to the low genetic variability within the genus Chara, it contains a total of six species occurring in Europe that cannot be distinguished by using rbcL or matK: C. hispida L., C. baltica, C. papillosa Kützing, C. aculeolata Kützing in Reichenbach, C. horrida and C. subspinosa Ruprecht. However, according to the local and the morphological criteria of the oospores, this cluster was assigned to the C. baltica/C. horrida group. In addition, a few individuals of C. connivens Salzmann ex A. Braun C. virgata and C. globularis were identified in the sediment samples of Breitling/Stove (Table ).
Table 5. Distribution of identified taxa from the 198 individual oospores based on rbcL and matK sequences.
The discrimination analysis revealed that only around 50% of the original grouped oospores were classified correctly based on their morphology. The oospores of the diplostichous C. hispida group showed a high morphological variability. Nearly 50% of the genetically assigned C. hispida oospores were misidentified on morphological characteristics. The results for the oospores of the C. aspera/C. canescens group and T. nidifica were different. Just a quarter of the oospores were confirmed by the genetic analyses. In contrast to the oospores of the C. hispida group, approximately 90% of the genetic C. canescens and T. nidifica oospores were also located in the morphological cluster (Table ).
Table 6. Number of cases correctly (bold) and incorrectly assigned to each of the three main groups based on the discrimination analysis: morphological determination of oospores compared with the genetic results.
Germination
With the exception of Michaelsdorf (the station with the lowest proportion of the intact diaspores) the germinations were observed at all the sample sites and sediment layers, with the highest abundances between 5 and 15 cm sediment depths (74%, Figure ). This result agrees with the depth profiles of the diaspore bank; the main volume of diaspores was found up to the 15 cm sediment depth. The sediment layers greater than 20 cm, often rich in clay and loam, revealed no germination potential.
Figure 6. Germination success: (a) The percentage of emerged seedlings from the analysed sediment layers. (b) The percentage of seedling abundance for the germinated taxa.
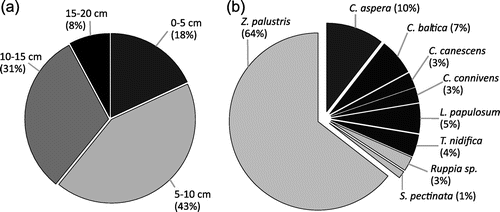
The seedlings of three angiosperms and six charophytes were identified. Z. palustris represented 64% of all observed seedlings, followed by C. aspera that revealed a germination success of 10% (Figure ). The rare taxa of the vegetation community emerged occasionally from the sediment: T. nidifica (Breitling/Stove), L. papulosum (Orth and Lehmkenhafen), C. connivens (Neuensiener Lake).
Due to the small number of individuals that emerged from the sediment, the germination rate of single taxa was determined only for Breitling/Stove and a combination of Orth & Lehmkenhafen. The obtained germination rates were quite variable for the analysed taxa (Table ). Comparably high germination rates were observed for Z. palustris and L. papulosum, whereas the values for the remaining taxa ranged from 0 to 1%. Moreover, the vegetative reproduction based on, for instance, bulbils or tubers, was an important component (Table ).
Table 7. Number of individuals emerged in the germination experiments with percentage of sexual reproduction from seeds or oospores in parentheses, density of intact diaspores in number per litre and relative germination rate (based only on sexual reproduction in parentheses) from the combined abundances of the sites Orth and Lehmkenhafen (salinity 14) and Breitling/Stove (salinity 11).
Discussion
Vegetation–diaspore relationship
This study demonstrates the clear differences in the species distribution of the recent vegetation and the diaspore bank along the Baltic coastline. The dominance of the angiosperms and the lack of the charophyte vegetation at nearly all the analysed sites are identical to the observations in other areas of the Baltic Sea (e.g. Pitkänen et al. Citation2013) and are mainly explained by the high eutrophication rate of the last decades (e.g. Munkes Citation2005; Schiewer Citation1997). The absence of the small charophytes in the turbid water is not due to a higher demand for light during photosynthesis (Chambers and Kaiff Citation1985). In clear waters, the charophytes are found at considerably greater depths than angiosperms (e.g. 60 m in Lake Tahoe according to Frantz and Cordone Citation1967). The distribution of both groups can be explained mainly by their different types of growth. The charophytes form short, bottom-dwelling patches, whereas the angiosperms, like S. pectinata, have the potential for pronounced elongation, and canopy-like growth in the turbid environments (Blindow Citation1992). Therefore, the charophytes are limited in the nutrient-rich turbid waters, mainly due to the lack of light caused by shading.
As directed by the selection of the sampling sites, we found different species compositions among the sites that are mostly associated with changing ranges of salinities. Species such as T. nidifica (salinity range: 8–25 according to Doege et al. Citation2016) and Z. marina (salinity range: 6–20 according to Salo, Pedersen, and Boström Citation2014) were found at the sites with the highest salinities such as Orth and Lehmkenhafen. Stuckenia pectinata (salinity range: 0–18 according to Luther Citation1951) or C. aspera (salinity range: 0–18 according to Doege et al. Citation2016), with broader salinity ranges, grew at nearly all sites. The occurrence of N. marina up to the salinities of 7 corresponded with the salinity range of 2–8 described by Eggert et al. (Citation2006).
In contrast to the recent vegetation, we found many more oospores than seeds of angiosperms in the sediment samples. Consistent with the findings of several studies (e.g. Blindow et al. Citation2016; Bonis and Lepart Citation1994; Steinhardt and Selig Citation2007, Citation2009, Citation2011), this result reflects many years of accumulation and burial (Casanova and Brock Citation1990). Bonis and Grillas (Citation2002) noted accordingly that the diaspore reservoir consists mainly of non-vital oospores. The differences between the recent macrophyte vegetation and the diaspore reservoirs are explained by the different life strategies (Kautsky Citation1988). The charophytes are among the first colonizing pioneers in the water bodies (Wade Citation1990) and mainly found in stressed habitats, benefiting from a high sexual reproduction with low germination rates and the dormant oospores that accumulate in the sediment (Kautsky Citation1988).
Within the charophyte fraction, we found Tolypella oospores as the dominating taxon, but rarely found it within the recent vegetation, a phenomenon regularly observed in the Baltic Sea (e.g. Steinhardt and Selig Citation2007). The individuals of this taxon are sometimes missed, because they germinate during the spring, and have a short vegetative time (Hamann and Becker Citation2016). However, Blindow et al. (Citation2016) proposed that the Tolypella oospores were deposited during the 1950s and 1960s, when this species was common at the “Westrügener Bodden”. The same could be assumed for the analysed sites of this study, as Tolypella used to be common along the coastline (Urbaniak Citation2003).
Furthermore, it is difficult to infer a pristine dominance of charophytes from a successful germination of oospores, since the fertility varies significantly (Acosta et al. Citation1999). Some species (e.g. T. nidifica) produce a very high number of oospores, while the dioecious species are only represented by the female part of the population (e.g. C. aspera). The information about the asexual reproduction by fragmentation or bulbils is not included in the diaspore bank.
The dominance of Z. palustris seeds (Figure (e)) is explainable by their shape: compared with the other higher plants, seeds of Z. palustris are flattened and easier to disperse (Grelsson and Nilsson Citation1991). The sexual propagules, such as R. cirrhosa (Figure (b)) or S. pectinata (Figure (a)), are heavier and more rounded, fall from the plant and usually accumulate directly under the recent vegetation (Kautsky Citation1988). Thus, the taxa with the heavy propagules seem to form stable populations in the habitats to which they are well adapted. In contrast, the taxa with the small and lightweight diaspores moved by hydrochory colonize new habitats.
The taxa S. pectinata and M. spicatum were abundant in the established vegetation, but rarely present in the sediment. For both taxa, the vegetative propagation is described as the most important strategy (Vári Citation2013). Van Wijk (Citation1989) revealed that the seed bank formation is not a consistent part of the regular survival strategy of S. pectinata, as the reproduction is mainly based on tubers. The highly competitive taxon M. spicatum shows a similar strategy, with a long period of vegetative growth (Kautsky Citation1988) and (auto)fragmentation (e.g. Hartleb, Madsen, and Boylen Citation1993). The fragmentation decreases with increasing salinity (Martin and Valentine Citation2014), which most probably restricts the distribution of M. spicatum up to a salinity of 9 in this study. At the Baltic coastline, the increasing salinity is commonly combined with the reduced eutrophication. As shown by Smith et al. (Citation2002), the less nutrient-loaded sediments lead to a significantly increased auto-fragment production of M. spicatum. Thus, a direct physiological control by salinity is very plausible. Additionally, sexual reproduction was not observed for the individuals that live close to their maximum salinity tolerance (Kautsky Citation1988).
Most diaspore bank data in the literature consist of samples taken down to the 5 cm sediment depth (e.g. Grelsson and Nilsson Citation1991; Steinhardt and Selig Citation2007); more rarely down to the 10 cm sediment depth (e.g. Kalin and Smith Citation2007). In this study, the different sediment layers, down to the 30 cm sediment depth, were analysed independently. Our results revealed the highest relative distribution of the (intact) diaspores from 5 to 15 cm sediment depth. The vertical structure is similar to the seed banks of meadows or swamps (Leck Citation1989), where the distribution of the diaspores is related to the sedimentation processes, which bury the seeds. The lower diaspore abundances of the uppermost layer could be explained by the sampling time between May and September, and reflect the germination output before the sampling season. The deposition and the resuspension processes of the sediment also influence the structure of the diaspore composition at the Baltic coast (Lass and Matthäus Citation2008) and are influenced by a variety of parameters: for instance sediment composition, waves, turbulences, as well as the vegetation density or bioturbation (Gogina et al. Citation2017).
Diaspore–germination relationship
Our results demonstrated that at all the sites the diaspores germinated under in situ-like conditions. The most emerged seedlings were observed from sediment at 5–15 cm depth, following the highest diaspore dispersal. Li et al. (Citation2008) revealed that the diaspores of the deeper sediment layers are conserved and form the macrophyte pool for the colonization processes, the diaspore banks being crucial for the maintenance and resilience of the plant communities (Brock et al. Citation2003). Several authors showed that the sexual propagules remain viable even for several decades (e.g. Rodrigo and Alonso-Guillén Citation2013) and that the restoration measures resulted in an increase of the charophyte growth, re-established from the oospore reservoir (Beltman and Allegrini Citation1997). Steinhardt and Selig (Citation2011) confirmed this assumption by successfully inducing germination after the sediment resuspension experiments for the coastal lakes at the Baltic Sea. In accordance with the earlier results, we showed that the composition of the emerged taxa differed remarkably from the recent vegetation of the analysed sites and that activating the diaspore reservoir could result in a shift of species composition.
The dominance of Z. palustris in the germination experiments of this study could be explained by the low amounts of sediment used. Spencer and Ksander (Citation2002) showed that the germination rates ranged from 76% for the seeds placed on the sediment surface, over 13% for the seeds placed at the 2 cm depth, to zero for the seeds buried at the 5 cm depth. Our comparable results between 4% and 17% were only relevant for the generative reproduction.
In contrast, the oospore germination rates fell into the range of 0–12%. Various factors, apart from light as an important trigger (de Winton, Casanova, and Clayton Citation2004), affect the germination of the dormant oospores. Temperature seems to control the germination of C. contraria oospores with an optimum between 24 and 27°C (Sabbatini et al. Citation1987). In our experiments, the germination rates of C. contraria Braun ex Kützing were not calculated, because no oospores were identified in the sediments. Steinhardt and Selig (Citation2011) showed that under comparable conditions, the germination rates of C. contraria oospores from the coastal lakes are in the range of 1–3%. The other factors, initiating the germination processes, include desiccation (Casanova and Brock Citation1996), seasonality (Stross Citation1989), or changing redox conditions (Kalin and Smith Citation2007). The germination rates from the natural sediments range between 5 and 50% (Bonis and Grillas Citation2002). These estimates are comparable with the results of this study, when both the vegetative and the sexual reproduction were taken into account. Considering the two reproductive modes, the species-specific strategies became apparent in this study: two-thirds of the C. aspera seedlings emerged from bulbils. The same result was observed for 100% of the C. baltica seedlings. Thus the production of bulbils is important for the short time survival (i.e. the winter period) of the taxa. Van den Berg, Coops and Simons (Citation2001) described a nearly 100% germination success of C. aspera bulbils. The importance of the vegetative propagules of the brackish water C. aspera was demonstrated by Blindow et al. (Citation2009). The authors revealed that only 8% of the gametangia of the brackish water females were developed to oospores. In contrast, the C. aspera oospore germination rates ranged between 1 and 15%, depending on the light level and the burial depth (van den Berg, Coops, and Simons Citation2001). Such low germination rates, combined with the accumulation processes in the sediment, support the idea that the oospores are adapted to a long time survival.
Genetic determination
There has been an increased focus on the taxonomic studies in the Characeae, based on the genetic applications in previous years (e.g. Nowak, Schubert, and Schaible Citation2016; Perez et al. Citation2014). In this study, the sequencing of the genetic markers from the individual oospores was successfully tested for the first time. It has to be noted that both the intact and defective oospores have shown positive PCR signals, although the length of the amplified regions seemed to be a critical factor. The dark oospore wall is a complex multilayered wall, composed of eight distinct layers (Leitch Citation1989). After the fertilization, the wall is derived partly from the oogonium cell and from the spiral cells, but the volume of the “living” cells is not a part of the oospore wall. Consequently, the PCR signal obtained must be derived from the DNA of the non-germinated oogonium cell inside the oospores.
Because of the huge variability within the taxa, the applicability of the oospore determination keys, especially for the brackish water material, is limited. We successfully combined the morphological analysis with the genetic determination. The oospores that were classified only to a group level (e.g. C. aspera/C. canescens group), could then be defined up to a species level. Generally, the regional distribution of T. nidifica oospores is used as an identification criterion: T. nidifica is a typical brackish water species in the Baltic Sea (Hamann and Becker Citation2016). However, T. glomerata (Desvaux) Leonhardi, as another brackish water taxon, could also grow near the Baltic Sea coast. However, T. glomerata is more commonly found in freshwater and is described in North Germany, but not directly for the Baltic Sea (van de Weyer Citation2016). The distinction of the oospores of both taxa is not possible using light microscopy, but the SEM studies of the oospore wall may be used for the differentiation (Urbaniak, Langangen, and van Raam Citation2012). Based on the rbcL sequences, the oospores of this study were clearly assigned to the T. nidifica specimens collected in Lehmkenhafen. The sequences clearly differed from T. glomerata data, obtained from GenBank (Perez et al. Citation2014). To test whether the brackish water Tolypella specimens of the Baltic Sea coast belong definitely to the taxon T. nidifica, additional molecular data should be gathered.
However, based on the sequence data, we identified the oospores of the diplostichous Hartmania-Cluster on a large scale. This cluster cannot be differentiated with the matK or the rbcL sequences (Nowak, Schubert, and Schaible Citation2016). The successful implementation indicates that a genetic approach may be useful for the future diaspore analyses. However, the development of more species-specific markers is an essential need for more frequent and general use.
Conclusion
The vegetation diversity and the diaspore bank composition of the German Baltic coast are mainly influenced by the salinity and eutrophication. The germination experiments revealed the existence of the vital oospores, even from rare species like L. papulosum or C. connivens. The search for at least single sites with consistent patterns of relationships between the diaspore abundance, the current vegetation and the germination was not successful. Overall, we observed low germination rates. Consequently, we questioned if this rate can be increased by collecting the samples during the different seasons or by simulating the germination conditions through pre-treatments, testing the role of temperature or light. The extent to which the nutrients or the microbiome of the sediments also influence the germination rate of the diaspores in the seed bank should be analysed in future projects. Another aspect, in our view, should be a causality analysis of the species composition, comparing the pristine vegetation, the diaspore bank and the emerged seedlings. The investigations on the higher plants (Gutterman and Gendler Citation2005) indicated not only an annual germination rhythm, but also positional effects between the terminal, central and basal seeds, even many years after the seed maturation. The extent to which the historical environmental conditions, the position of the seeds/oospores during maturation, or their age, influences the reproductive success is unknown for the aquatic plants. If the germination success of a species in a single year follows historical programmes, the current environmental conditions could partly represent a reflection of conditions in the seed maturity. Although the natural periodicity is very complex, we often assume that similar environmental conditions provide programmes for the reproductive success under similar conditions. However, the diaspore reservoir is an extraordinarily complex system. Its use for the large-scale restoration projects still needs more information. The seasonality, the dormancy and the time scales are suggested as the keywords for the respective investigations in the future.
Nevertheless, the indigenous diaspore reservoirs of the sediment provide the best preconditions to re-introduction of the vital macrophyte communities that are well adapted to the local ecological conditions. We can conclude that the diaspore bank represents a substantial component of the plant community at the Baltic Sea coastline in spite of the generally intensive sediment transport.
Disclosure statement
We confirm that there are no known conflicts of interest associated with this publication.
Funding
The project was financially supported by the “Forschungsstiftung Ostsee” (07/2013) and partly by the European Regional Development Fund (ERDF, UHRO26).
Notes on contributors
Petra Nowak (corresponding author) is a marine biologist specializing in the biodiversity and evolution of macroalgae and has been conducting research for 7 years at the University of Rostock. Beside the taxonomical aspect, she is focussed on the molecular and the morphological approaches towards understanding the acclimation and adaptation processes of this group. Contribution: research question, data analysis, interpretation of results, manuscript preparation.
Tim Steinhardt was an aquatic ecologist at the University of Rostock specialized in the submerged macrophyte vegetation of coastal ecosystems. Since 2001, he has concentrated on distribution and regeneration potential of diaspores with a focus on the ecological status of inner coastal waters. Contribution: general concept and research question, data analysis, interpretation of results.
Ulla von Ammon is a marine biologist specializing in the systematics of algae and metabarcoding studies on the marine invasive biofouling. She is doing her PhD in a collaborative project of the University of Auckland, the Cawthron Institute in Nelson and the National Institute of Water and Atmospheric Research, New Zealand. Contribution: genetic data analysis, interpretation of results, manuscript preparation.
Henning Rohde is a landscape ecologist specializing in the aquatic ecology and working at the Department for Environmental Protection at the municipality of Rostock. Contribution: data analysis, interpretation of results, manuscript preparation.
Arne Schoor is a marine ecologist employed at the University of Rostock with 20 years of experience in the aquatic ecology, nutrient cycling, molecular acclimation and stress physiology of algae. Contribution: research question, technical solutions and implementation, interpretation of results, manuscript preparation.
Anja Holzhausen has finished her PhD in 2016. Since 2016 she has been employed as Research Associate and as a project member at the University of Rostock. She has been conducting research for 4 years on the ecology, phenology and physiology of the Characeae of brackish and fresh water. Contribution: research question, interpretation of results, manuscript preparation.
Ralf Schaible has a strong background in the aquatic ecology and evolution, including adaptive and life-history evolution, has worked with macrophytes (Characeae) and invertebrates (freshwater polyp, mussels, social insects) in the field and in the laboratory. Contribution: research question, interpretation of results.
Hendrik Schubert is a marine biologist specializing in the aquatic ecology with a focus on ecophysiology of autotrophs. Over the last 15 years, he has been Professor for Ecology at the University of Rostock, investigating acclimation of organisms to the brackish conditions of the Baltic Sea and unravelling the trophic interactions and the seasonality of the coastal ecosystems. Contribution: general concept and research question, data analysis, interpretation of results, manuscript preparation.
Supplemental data
The supplemental material for this paper is available online at https://doi.org/10.1080/23818107.2017.1400464
TABG_1400464_TableS1.doc
Download MS Word (46 KB)Acknowledgements
We thank Michael Kolberg and Steffen Sprenger for their help during the field work. The authors are very grateful to the two anonymous reviewers and the editors, Carles Martin Closas and Elisabeth Dodinet, for the critical reading and the suggestions, which helped us to improve the manuscript. We also thank Mary Beilby for thorough language editing.
References
- Abramoff, M. D., P. J. Magalhães, and S. J. Ram. 2004. “Image Processing with ImageJ.” Biophotonics International 11 (7): 36–42.
- Acosta, L. W., M. R. Sabbatini, O. A. Fernández, and M. A. Burgos. 1999. “Propagule Bank and Plant Emergence of Macrophytes in Artificial Channels of a Temperate Irrigation Area in Argentina.” Hydrobiologia 415: 1–5.10.1023/A:1003808713907
- Barett, S. C., and J. R. Kohn. 1991. “Genetic and Evolutionary Consequences of Small Population Size in Plants: Implications for Conservation.” In Genetics and Conservation of Rare Plants, edited by D. A. Falk and K. E. Holsinger, 3–30. New York: Oxford University Press.
- Beijernick, W. 1947. Zadenatlas der nederlandsche flora [Seed atlas of Dutch flora]. Veenman: Wageningen.
- Beltman, B., and C. Allegrini. 1997. “Restoration of Lost Aquatic Plant Communities: New Habitats for Chara.” Netherlands Journal of Aquatic Ecology 30 (4): 331–337.10.1007/BF02085876
- van den Berg, M. S., H. Coops, and J. Simons. 2001. “Propagule Bank Buildup of Chara aspera and its Significance for Colonization of a Shallow Lake.” Hydrobiologia 462 (1): 9–17.10.1023/A:1013125603555
- Bertsch, K. 1941. Früchte und Samen: Ein Bestimmungsbuch zur Pflanzenkunde der vorgeschichtlichen Zeit [Fruits and Seeds: A Determination Book for Plant Science of Prehistoric Times]. Stuttgart: Ferdinand Enke.
- Blindow, I. 1992. “Decline of Charophytes during Eutrophication: Comparison with Angiosperms.” Freshwater Biology 28 (1): 9–14.10.1111/fwb.1992.28.issue-1
- Blindow, I., N. Möllmann, M. G. Boegle, and M. Schütte. 2009. “Reproductive Isolation in Chara aspera Populations.” Aquatic Botany 91 (3): 224–230.10.1016/j.aquabot.2009.06.009
- Blindow, I., S. Dahlke, A. Dewart, S. Flügge, M. Hendreschke, A. Kerkow, and J. Meyer. 2016. “Long-Term and Interannual Changes of Submerged Macrophytes and their Associated Diaspore Reservoir in A Shallow Southern Baltic Sea Bay: Influence of Eutrophication and Climate.” Hydrobiologia 778 (1): 121–136.10.1007/s10750-016-2655-4
- Bonis, A., and P. Grillas. 2002. “Deposition, Germination and Spatio-Temporal Patterns of Charophyte Propagule Banks: A Review.” Aquatic Botany 72 (3–4): 235–248.10.1016/S0304-3770(01)00203-0
- Bonis, A., and J. Lepart. 1994. “Vertical Structure of Seed Banks and the Impact of Depth of Burial on Recruitment in Two Temporary Marshes.” Vegetatio 112 (2): 127–139.10.1007/BF00044687
- Braun-Blanquet, J. 1964. Pflanzensoziologie - Grundzüge der Vegetationskunde [Plant Socology - Principles of Vegetation Science]. Wien: Springer.
- Brock, M. A., D. L. Nielsen, R. J. Shiel, J. D. Green, and J. D. Langley. 2003. “Drought and Aquatic Community Resilience: The Role of Eggs and Seeds in Sediments of Temporary Wetlands.” Freshwater Biology 48 (7): 1207–1218.10.1046/j.1365-2427.2003.01083.x
- Casanova, M. T., and M. A. Brock. 1990. “Charophyte Germination and Establishment from the Seed Bank of an Australian Temporary Lake.” Aquatic Botany 36 (3): 247–254.10.1016/0304-3770(90)90038-M
- Casanova, M. T., and M. A. Brock. 1996. “Can Oospore Germination Patterns Explain Charophyte Distribution in Permanent and Temporary Wetlands?” Aquatic Botany 54 (4): 297–312.10.1016/0304-3770(96)01032-7
- Chambers, P. A., and J. Kaiff. 1985. “Depth Distribution and Biomass of Submersed Aquatic Macrophyte Communities in Relation to Secchi Depth.” Canadian Journal of Fisheries and Aquatic Sciences 42 (4): 701–709.10.1139/f85-090
- Doege, A., K. van de Weyer, R. Becker, and H. Schubert. 2016. “Kapitel 8 Bioindikation mit Characeen [Bioindication with Characeae].” In Armleuchteralgen. Die Characeen Deutschlands [Stoneworts. Characeae of Germany], edited by A. G. Chara, 97–138. Berlin Heidelberg: Springer.
- Eggert, A., S. Ihnken, U. Selig, U. Karsten, and H. Schubert. 2006. “Distribution of Three Submersed Macrophytes in Coastal Lagoons of the German Baltic Sea: Comparison of Laboratory and Field Data.” Botanica Marina 49 (5/6): 386–395.
- Ellenberg, H. 1992. Zeigerwerte von Pflanzen in Mitteleuropa [Indicative Values of Plants in Central Europe]. Göttingen: Erich Goltze.
- European Union. 2000. “Directive 2000/60/EC of the European Parliament of the Council of 23 October 2000 establishing a framework for Communities in the field of water policy.” Official Journal of the European Communities L327: 1–73.
- Frantz, T. C., and A. J. Cordone. 1967. “Observations on Deepwater Plants in Lake Tahoe, California and Nevada.” Ecology 48 (5): 710–714.
- Gogina, M., C. Morys, S. Forster, U. Gräwe, R. Friedland, and M. L. Zettler. 2017. “Towards Benthic Ecosystem Functioning Maps: Quantifying Bioturbation Potential in the German Part of the Baltic Sea.” Ecological Indicators 73: 574–588.10.1016/j.ecolind.2016.10.025
- Grelsson, G., and C. Nilsson. 1991. “Vegetation and Seed-Bank Relationships on a Lakeshore.” Freshwater Biology 26 (2): 199–207.10.1111/fwb.1991.26.issue-2
- Gutterman, Y., and T. Gendler. 2005. “Annual Rhythm of Germination of Seeds of Mesembryanthemum nodiflorum 32 years after Collection.” Seed Science Research 15 (3): 249–253.10.1079/SSR2005215
- Hamann, U., and R. Becker. 2016. “Kapitel 12.36 Tolypella nidifica.” In Die Characeen Deutschlands Armleuchteralgen [Stoneworts. Characeae of Germany], edited by A. G. Chara, 529–538. Berlin Heidelberg: Springer.
- Hartleb, C. F., J. D. Madsen, and C. W. Boylen. 1993. “Environmental Factors Affecting Seed Germination in Myriophyllum spicatum L.” Aquatic Botany 45 (1): 15–25.10.1016/0304-3770(93)90049-3
- Jeppesen, E., M. Søndergaard, M. Meerhoff, T. L. Lauridsen, and J. P. Jensen. 2007. “Shallow Lake Restoration by Nutrient Loading Reduction–Some Recent Findings and Challenges Ahead.” Hydrobiologia 584 (1): 239–252.10.1007/s10750-007-0596-7
- Kalin, M., and M. P. Smith. 2007. “Germination of Chara vulgaris and Nitella flexilis Oospores: What are the Relevant Factors Triggering Germination?” Aquatic Botany 87 (3): 235–241.10.1016/j.aquabot.2007.06.004
- Kautsky, L. 1988. “Life Strategies of Aquatic Soft Bottom Macrophytes.” Oikos 53 (1): 126–135.10.2307/3565672
- Krause, W. 1997. “Charales (Charophyceae).” In Süßwasserflora von Mitteleuropa [Freshwater flora of Central Europe], edited by A. Ettl, G. Gärtner, H. Heynig, and D. Mollenhauer, 202. Jena: G. Fischer.
- Krause-Jensen, D., S. Sagert, H. Schubert, and C. Boström. 2008. “Empirical Relationships Linking Distribution and Abundance of Marine Vegetation to Eutrophication.” Ecological Indicators 8 (5): 515–529.10.1016/j.ecolind.2007.06.004
- Lass, H.-U., and W. Matthäus. 2008. “General Oceanography of the Baltic Sea.” In State and Evolution of the Baltic Sea, 1952-2005. A Detailed 50-Year Survey of Meteorology and Climate, Physics, Chemistry, Biology, and Marine Environment, edited by R. Feistel, G. Nausch, and N. Wasmund, 5–44. Hoboken: JWiley.
- Leck, M. A. 1989. “Wetland Seed Banks.” In Ecology of Soil Seed Banks, edited by M. A. Leck, V. T. Parker, and R. L. Simpson, 283–305. San Diego, CA: Academic Press.
- Leitch, A. R. 1989. “Formation and Ultrastructure of a Complex, Multilayered Wall around the Oospore of Chara and Lamprothamnium (Characeae).” British Phycological Journal 24 (3): 229–236.10.1080/00071618900650251
- Li, E.-H., G.-H. Liu, W. Li, L.-Y. Yuan, and S.-C. Li. 2008. “The Seed-Bank of a Lakeshore Wetland in Lake Honghu: Implications for Restoration.” Plant Ecology 195 (1): 69–76.10.1007/s11258-007-9299-4
- Luther, H. 1951. “Verbreitung und Ökologie der höheren Wasserpflanzen im Brackwasser der Ekenäs-Gegend in Südfinnland. II. Spezieller Teil [Distribution and Ecology of Higher Aquatic Plants in Brackish Waters in the Ekenäs Region of southern Finland].” Acta Botanica Fennica 50: 1–370.
- Martin, C. W., and J. F. Valentine. 2014. “Sexual and Asexual Reproductive Strategies of Invasive Eurasian milfoil (Myriophyllum spicatum) in Estuarine Environments.” Hydrobiologia 727 (1): 177–184.10.1007/s10750-013-1798-9
- Munkes, B. 2005. “Eutrophication, Phase Shift, the Delay and the Potential Return in the Greifswalder Bodden, Baltic Sea.” Aquatic Sciences 67 (3): 372–381.10.1007/s00027-005-0761-x
- Nowak, P., H. Schubert, and R. Schaible. 2016. “Molecular Evaluation of the Validity of the Morphological Characters of Three Swedish Chara Sections: Chara, Grovesia, and Desvauxia (Charales, Charophyceae).” Aquatic Botany 134 (1): 113–119.10.1016/j.aquabot.2016.08.001
- Perez, W., J. Hall, R. McCourt, and K. G. Karol. 2014. “Phylogeny of North America Tolypella (Charophyceae, Charophyta) based on Plastid DNA Sequences with a Description of Tolypella ramosissima sp. nov.” Journal of Phycology 50 (5): 776–789.10.1111/jpy.2014.50.issue-5
- Pitkänen, H., M. Peuraniemi, M. Westerbom, M. Kilpi, and M. v. Numers. 2013. “Long-Term Changes in Distribution and Frequency of Aquatic Vascular Plants and Charophytes in An Estuary in the Baltic Sea.” Annales Botanici Fennici 50: 1–54.10.5735/085.050.701
- Rodrigo, M. A., and J. L. Alonso-Guillén. 2013. “Assessing the Potential of Albufera de València Lagoon Sediments for the Restoration of Charophyte Meadows.” Ecological Engineering 60: 445–452.10.1016/j.ecoleng.2013.09.041
- Rothmaler, W., R. Schubert, and W. Vent. 1994. Exkursionsflora von Deutschland - Gefäßpflanzen: Kritischer Band [Field flora of Germany - Vascular Plants: Critical Volume]. Jena: Gustav Fischer.
- Sabbatini, M. R., J. A. Argüello, O. A. Fernández, and R. A. Bottini. 1987. “Dormancy and Growth-Inhibitor Levels in Oospores of Chara contraria A. Braun ex Kütz. (Charophyta).” Aquatic Botany 28 (2): 189–194.10.1016/0304-3770(87)90040-4
- Sagert, S., U. Selig, and H. Schubert. 2008. “Phytoplanktonindikatoren zur ökologischen Klassifizierung der deutschen Küstengewässer der Ostsee [Phytoplankton Indicators for Ecological Classification of Coastal Waters along the German Baltic Coast].” Rostocker Meeresbiologische Beiträge 20: 45–69.
- Salo, T., M. F. Pedersen, and C. Boström. 2014. “Population Specific Salinity Tolerance in Eelgrass (Zostera marina).” Journal of Experimental Marine Biology and Ecology 461: 425–429.10.1016/j.jembe.2014.09.010
- Schernewski, G., and U. Schiewer. 2002. “Status, Problems and Integrated Management of Baltic Coastal Ecosystems.” In Baltic Coastal Ecosystems, edited by G. Schernewski and U. Schiewer, 1–16. Berlin: Springer.10.1007/978-3-662-04769-9
- Schiewer, U. 1997. “30 years’ Eutrophication in Shallow Brackish Waters - Lessons to be Learned.” Hydrobiologia 363 (1/3): 73–79.10.1023/A:1003194226294
- Schiewer, U. 2008. “Darß-Zingst Boddens, Northern Rügener Boddens and Schlei.” In Ecology of Baltic Coastal Waters. Ecological Studies (Analysis and Synthesis), edited by U. Schiewer, 35–81. Berlin: Springer.10.1007/978-3-540-73524-3
- Schlungbaum, G., and H. Baudler. 1998. Erfordernisse, Möglichkeiten und Grenzen für eine nachhaltige Verbesserung der Gewässerbeschaffenheit in der Darss-Zingster Boddenkette [Requirements, Possibilities and Borders for a Sustainable Improvement of the Water Quality at the Darss-Zingster Bodden Chain]. Stralsund: Studie im Auftrag des BAUN-MV, LAUN-MV und STAUN.
- Schubert, H., P. Feuerpfeil, R. Marquardt, I. V. Telesh, and S. O. Skarlato. 2011. “Macroalgal Diversity along the Baltic Sea Salinity Gradient Challenges Remane’s Species-Minimum Concept.” Marine Pollution Bulletin 62 (9): 1948–1956.10.1016/j.marpolbul.2011.06.033
- Selig, U., A. Eggert, D. Schories, M. Schubert, C. Blümel, and H. Schubert. 2007. “Ecological Classification of Macroalgae and Angiosperm Communities of Inner Coastal Waters in the Southern Baltic Sea.” Ecological Indicators 7 (3): 665–678.10.1016/j.ecolind.2006.07.006
- Selig, U., T. Steinhardt, and H. Schubert. 2009. “Interannual Variability of Submerged Vegetation in a Brackish Coastal Lagoon on the Southern Baltic Sea.” Ekológia 28 (4): 412.10.4149/ekol_2009_04_412
- Smith, D. H., J. D. Madsen, K. L. Dickson, and T. L. Beitinger. 2002. “Nutrient Effects on Autofragmentation of Myriophyllum spicatum.” Aquatic Botany 74 (1): 1–17.10.1016/S0304-3770(02)00023-2
- Spencer, D. F., and G. G. Ksander. 2002. “Sedimentation Disrupts Natural Regeneration of Zannichellia palustris in Fall River, California.” Aquatic Botany 73 (2): 137–147.10.1016/S0304-3770(02)00016-5
- Steinhardt, T., and U. Selig. 2007. “Spatial Distribution Patterns and Relationship between Recent Vegetation and Diaspore Bank of a Brackish Coastal Lagoon on the Southern Baltic Sea.” Estuarine, Coastal and Shelf Science 74 (1–2): 205–214.10.1016/j.ecss.2007.04.004
- Steinhardt, T., and U. Selig. 2009. “Comparison of Recent Vegetation and Diaspore Banks along Abiotic Gradients in Brackish Coastal Lagoons.” Aquatic Botany 91 (1): 20–26.10.1016/j.aquabot.2009.01.004
- Steinhardt, T., and U. Selig. 2011. “Influence of Salinity and Sediment Resuspension on Macrophyte Germination in Coastal Lakes.” Journal of Limnology 70 (1): 11–20.10.4081/jlimnol.2011.11
- Stross, R. G. 1989. “The Temporal Window of Germination in Oospores of Chara (Charophyceae) following Primary Dormancy in the Laboratory.” New Phytologist 113 (4): 491–495.10.1111/nph.1989.113.issue-4
- Telesh, I. V., H. Schubert, and S. O. Skarlato. 2013. “Life in the Salinity Gradient: Discovering Mechanisms behind a New Biodiversity Pattern.” Estuarine, Coastal and Shelf Science 135: 317–327.10.1016/j.ecss.2013.10.013
- Urbaniak, J. 2003. “Chapter 4.29 Tolypella nidifica (O. F. Müll.).” In Charophytes of the Baltic Sea, edited by H. Schubert and I. Blindow, 223–233. Königstein: Gantner.
- Urbaniak, J., A. Langangen, and J. C. van Raam. 2012. “Oospore Wall Ornamentation in the Genus Tolypella (Charales, Charophyceae).” Journal of Phycology 48 (6): 1538–1545.10.1111/jpy.12007
- Vári, Á. A. B. 2013. “Colonisation by Fragments in Six Common Aquatic Macrophyte Species.” Fundamental and Applied Limnology 183 (1): 15–26.10.1127/1863-9135/2013/0328
- Vedder, F. 2004. “Morphologie und Taxonomie rezenter und subfossiler Characeen-Oosporen aus der Ostsee [Morphology and Taxonomy of Recent and Subfossil Oospores of Charophytes out of the Baltic Sea].” Rostocker Meeresbiologische Beiträge 13: 43–54.
- Wade, P. M. 1990. “The Colonisation of Disturbed Freshwater Habitats by Characeae.” Folia Geobotanica et Phytotaxonomica 25 (3): 275–278.10.1007/BF02913027
- van de Weyer, K. 2016. “Kapitel 12.34 Tolypella glomerata.” In Armleuchteralgen. Die Characeen Deutschlands [Stoneworts. Characeae of Germany], edited by A. G. Chara, 514–522. Berlin: Springer.
- van Wijk, R. J. 1989. “Ecological Studies on Potamogeton pectinatus L. III. Reproductive Strategies and Germination Ecology.” Aquatic Botany 33 (3): 271–299.10.1016/0304-3770(89)90042-9
- de Winton, M. D., M. T. Casanova, and J. S. Clayton. 2004. “Charophyte Germination and Establishment under Low Irradiance.” Aquatic Botany 79 (2): 175–187.10.1016/j.aquabot.2004.01.013