Abstract
Perianth dimorphism in Nigella damascena (Ranunculaceae) has been documented for four centuries, and its genetic basis started to be studied almost a century ago. Based on herbarium collections, we reconstructed the evolution of the distribution area of the wild-type (perianth composed of a calyx and nectariferous petals) and mutant (apetalous) floral morphs in mainland France over the last two centuries. We showed that the collection localities of the wild-type morph are mostly concentrated in the southern coastal areas of France, whereas the collection localities of the mutant morph were less numerous but more scattered in France. The collection effort of both morphs peaked in the second half of the nineteenth century and continuously decreased afterwards. Results are interpreted taking into account the biases in the constitution of a herbarium collection. Knowledge about the spatial distribution and the frequency of occurrence of wild-type and mutant populations through time will help botanists and evolutionary biologists investigate the origin and ecological grounds for the maintenance and propagation of the apetalous morph in the wild.
Introduction
Floral dimorphism has intrigued botanists and evolutionary biologists for centuries. Darwin devoted a book to this topic, focusing on heterostylous Primula flowers (Darwin Citation1897). Nigella damascena L. (Ranunculaceae), or love-in-a-mist, is a herbaceous species mainly distributed in the Mediterranean Basin, of which ancient use by human populations is well documented (Heiss and Oeggl Citation2005). In this species, two types of flowers are known at least since the end of the sixteenth century (Clusius Citation1601). The wild-type flower has a perianth composed of a pentamerous petaloid calyx and a series of 5–10 pouch-shaped nectariferous petals. By contrast, the unipartite perianth of the mutant type consists of numerous petaloid tepals (Deroin et al. Citation2015; Jabbour et al. Citation2015). The monogenic control of the dimorphism was first established by Toxopeus (Citation1927) based on segregation analyses, and its molecular basis was unraveled almost one hundred years later (Gonçalves et al. Citation2013; Zhang et al. Citation2013). For simplicity, we will adopt the terminology of Gonçalves et al. (Citation2013) to designate the wild-type (homozygous dominant or heterozygous for the combination of alleles controlling the dimorphism) and mutant (homozygous recessive) floral morphs, called [P] and [T], respectively. Human selection for plants with conspicuous flowers harboring supernumerary petaloid organs likely played an important role in the long-term maintenance of the [T] phenotype, but [T] populations are also expected to occur in the wild because individuals are self-fertile and plants can easily escape from gardens and become naturalized in favorable environments.
Herbarium collections are repositories of past and current plant biodiversity. Compiling the data held by all the herbarium specimens of a species allows reconstructing the evolution of its distribution area through time. In the case of N. damascena, knowledge about the spatial distribution and the frequency of occurrence of [P] and [T] populations through time would help botanists and evolutionary biologists investigate the origin and ecological grounds for the maintenance and propagation of the [T] morph in the wild. It should be possible to examine whether [T] populations can persist per se in the wild or if they are consistently found in the neighborhood of towns and villages, suggesting constant gene flow from gardens; alternatively, [T] individuals could consistently be found in the vicinity of [P] individuals, suggesting that the presence of nectariferous flowers in [P] plants may favor pollination and reproductive success of [T] plants with flowers lacking pollinator reward, and/or that the mutation is slightly deleterious and preferentially maintained in the heterozygous state. Another use of distribution maps would be to identify localities where N. damascena individuals from each morph could be currently collected.
To get insight into these topics, we built a quasi-exhaustive database of the world collection of N. damascena herbarium specimens. For this special issue of Botany Letters, we present the results concerning mainland France, explaining the database structure and the protocol for selecting the herbarium specimens for georeferencing and building the temporal sequence of distribution maps. We observe different distributions of the two morphs in mainland France, and discuss possible causes.
Materials and methods
Data collection and database construction
We built a database gathering together the information found on herbarium specimens of N. damascena (Figure ) from 39 herbaria, from the Mediterranean Basin or from other regions that house large amounts of Mediterranean species or species growing in areas with a Mediterranean climate. The herbaria sent specimens on loan to P (Paris Herbarium), where they were studied. They were photographed for the record. Alternatively, the herbaria sent images of their collection of N. damascena.
Figure 1. Herbarium material providing data about the floral morph and the collection date and locality. (A) Herbarium specimen of Nigella damascena (barcode P02575070) presenting individuals from the [P] floral morph. (B) A focus on the most mature flower. The white arrows point at two of the petals. (C) A focus on the label showing the collection number, the determination provided by the collector, the collection locality and date, and the name of the collector. (D) A flower from the [T] morph, from the specimen P00195185. Digital images of both specimens can be downloaded using the following links: http://coldb.mnhn.fr/catalognumber/mnhn/p/p02575070 and http://coldb.mnhn.fr/catalognumber/mnhn/p/p00195185.
![Figure 1. Herbarium material providing data about the floral morph and the collection date and locality. (A) Herbarium specimen of Nigella damascena (barcode P02575070) presenting individuals from the [P] floral morph. (B) A focus on the most mature flower. The white arrows point at two of the petals. (C) A focus on the label showing the collection number, the determination provided by the collector, the collection locality and date, and the name of the collector. (D) A flower from the [T] morph, from the specimen P00195185. Digital images of both specimens can be downloaded using the following links: http://coldb.mnhn.fr/catalognumber/mnhn/p/p02575070 and http://coldb.mnhn.fr/catalognumber/mnhn/p/p00195185.](/cms/asset/10b55ad6-e163-489a-bdd6-c8b7263cdcda/tabg_a_1422437_f0001_oc.jpg)
The data gathered from the biological specimen (Figure A–B and D) and from the label (Figure C) are organized into 16 fields: (A) herbarium code; (B) barcode or reference in the herbarium; (C) collector’s name; (D) collection number; (E–G) day, month, and year of collection; (H) country of origin, transcribed from the label or interpreted; current country names are provided; (I) collection locality; (J) other notes on the label (in the original language); (K) label mentioning that the specimen was in cultivation at the time of collection (yes/no); (L) assumption that the specimen was in cultivation at the time of collection (yes/no); (M) presence of flower(s) (yes/no); (N) flower morph (P/T, Figure B and D); (O) presence of capsule(s) (yes/no); (P) GPS coordinates in decimal degrees, in the WGS 84 system. In some cases, GPS coordinates were available directly from the label. In most cases, localities were georeferenced by us using Google Maps (Google Inc., Mountain View, CA, USA). The collection locality could be precisely specified on the label (e.g. “Gorges d’Estrabols, commune de Salvagnac, canton de Villeneuve”), allowing precise GPS coordinates to be determined (in red in Supplementary Table 2). Less precisely described collection sites, for example just mentioning the closest city, were also attributed GPS coordinates (in blue in Supplementary Table 2). In other cases, e.g. when only the country of origin is specified or when the plant was in cultivation when collected with no mention of the seed origin, localization data were considered unavailable and (P) cells were marked with a double-slash (//). Except for (H) and (L), all data are transcribed from the label without interpretation or come from the observation of the specimen. Label data were provided as a csv file by the following herbaria: BC, BEI, BM, E, FR, HUJ, and MA; it was double-checked before being included in our database.
Diachronic study of the distribution of Nigella damascena in France
For the current study, we focused on data relative to all specimens collected in France (654 specimens; Supplementary Table 1, Figure ), recorded from 20 herbaria (B (Berlin), BC (Barcelona), BEI (Beirut), BM (London), CAI (Cairo), E (Edinburgh), FI (Florence), FR (Frankfurt), G (Geneva), HUJ (Jerusalem), K (Kew), MA (Madrid), MEL (Melbourne), MPU (Montpellier), P (Paris), PAL (Palermo), S (Stockholm), TO (Torino), UPS (Uppsala), W (Vienna)); herbarium codes follow Thiers (Citation2017). For the analysis of the distribution of the two morphs over time, we did not consider specimens without flowers (or with flowers compressed in such a way that the floral morph was not distinguishable), duplicate specimens (belonging to the same collection, i.e. same collector and collection number) and specimens with no collection year. We considered two datasets extracted from the database:
| (i) | the georeferenced dataset excludes specimens with a double slash in (P), and gathered 409 specimens from 18 herbaria (all specimens from BEI and CAI herbaria were discarded). Among these specimens, 12 were composed of two different collections (different combination [collector + collection number]). After splitting these accessions into two, the final dataset consisted of 421 entries. Supplementary Table 2 shows the collections that were used to build the distribution maps. We classified the entries into four time intervals ([1800–1849], [1850–1899], [1900–1949], [1950–1999]), and into two categories ([P] and [T] morph) within each interval. When the collection date is an interval, the latest year was considered. After the sorting process described above, no specimen collected in France after 2000 was included in the dataset. Maps were built using SimpleMappr (Shorthouse Citation2010). | ||||
| (ii) | the [P]/[T] dataset was built to compare the number of collected specimens from each morph among each 50-year period. It includes the cultivated specimens and the specimens that could not be georeferenced, and comprises 469 entries. | ||||
Results
More than two centuries of biogeographical data captured in herbarium specimens
The oldest and most recent French specimens of N. damascena included in our database were collected in 1784 by an unknown collector and in 2015 by F. Jabbour, respectively. These specimens were both cultivated (in “Jardin de Sally” and in Gif-sur-Yvette (see Jabbour et al. Citation2015), respectively), and hence not georeferenced. The whole dataset consists of 654 collections. The three herbaria that contributed most in terms of the number of collections were P (Paris, 313 collections), MPU (Montpellier, 105) and G (Geneva, 43). The floral morph could be identified on 469 specimens (371 [P] and 98 [T]) and 421 specimens (352 [P] and 69 [T]) could be georeferenced. The three most active collectors of N. damascena specimens in France were Elisée Reverchon (1835–1914; 11 specimens), Florian Jabbour (1983–; nine specimens), and Brother Sennen (Etienne Marcellin Granié-Blanc, 1861–1937; eight specimens).
The collection localities of the [P] morph are mostly concentrated in the southern coastal areas of France
Figure shows the evolution of the distribution area of both floral morphs of N. damascena since 1800. Individuals from the [P] morph have mostly been collected from the southern half of France. Collection localities for this morph are particularly concentrated in the coastal areas of SE France (Provence-Alpes-Côte d’Azur, Languedoc-Roussillon and Corsica regions) and SW (Poitou-Charentes region). In the second half of the nineteenth century, four areas from which [P] specimens are mostly collected could be identified, namely, from West to East, the Charente-Maritime department, the Hérault department, the Rhône Valley and the Alpes-Maritimes department. For the [T] morph, the collection localities are less numerous but more scattered than for the [P] morph. Additionally, they tend to be more northerly distributed in France.
A peak of collection in the second half of the nineteenth century
The collection of both morphs was the highest between 1850 and 1899 (233, about half of the specimens of the dataset), before decreasing continuously afterwards (Figure A). The peak also coincides with more individuals from both morphs collected from the northern half of France. The collection of cultivated specimens, even though less numerous, follows the same temporal trend as the wild ones (Figure B). In the wild collections, [P] morph individuals are more frequent than [T] morph ones in all time intervals (2.6 to 10 times more). The proportion of [T] morph individuals was the highest in the first half of the twentieth century, reaching 28%. In contrast to the wild collection, more [T] than [P] individuals were found in the cultivated collection at each time period (Figure B). During the last two centuries, 28 of the c. 300 collectors of N. damascena collected both floral morphs, but only 15 of them collected both morphs in the same locality.
Discussion
Caution required when analyzing herbarium collection records
A collection of natural history is, by nature, biased. Not all the biodiversity can be captured in time and space, and the representativeness of a collection depends on the spatio-temporal uniformity of the collection effort (Auderset Joye, Castella, and Lachavanne Citation2002). Many collectors collect objects of natural history in the places where they live or travel, and they can be biased towards or against unusual objects (e.g. with an unusual morphology or spatial occurrence). They can collect, with priority, individuals with unusual phenotypes in order to have a record in their own collection, or they can ignore them because they deviate from the standard morphology. Indeed, botanists can purposely ignore plants considered as weeds and plants that are locally common. Hence, a herbarium collection can paradoxically lack plants that are common or present at a given locality for a long time. Knowing these limitations, herbarium collection data should not be over-interpreted. They give a picture of biodiversity at certain times and places, filtered by collectors’ interest that may vary according to education and societal context. For a given species, a way to partly circumvent bias is to consider the most exhaustive set of herbarium collections from the highest number of herbaria. This is what we intended to do for N. damascena.
The evolution of the Nigella damascena collection effort in France
In France, the collection effort concerning N. damascena has not been uniform over the last two centuries. After a peak in the second half of the nineteenth century, the number of specimens added to natural history collections decreased continuously. Does it reveal that the N. damascena distribution area has expanded and then shrunk, or that the collection effort has been lower during the last decades? We tend to favor the second hypothesis. Indeed, we cannot figure out any natural cause that could explain a decrease in abundance in the wild, since this species has been common in France (see SIFlore and INPN databases; Just et al. Citation2015; Muséum national d’Histoire Naturelle Citation2003–2017; Figure ). However, the decrease in specimen number collected in the first half of the twentieth century could be due to the impact of the two world wars on the activity of the scientists (Le Bras et al. Citation2017). Furthermore, nowadays we witness a general decline in collecting due to less funding for research in the field of natural history and the societal perception that collecting local flora is unnecessary (Renner and Rockinger Citation2016).
Figure 3. Frequency of [P] and [T] morphs of Nigella damascena in a Museum time-series spanning two centuries. (A) Wild specimens. (B) Cultivated specimens.
![Figure 3. Frequency of [P] and [T] morphs of Nigella damascena in a Museum time-series spanning two centuries. (A) Wild specimens. (B) Cultivated specimens.](/cms/asset/0bf989f9-a91e-4913-8534-fd5ef4e79bd1/tabg_a_1422437_f0003_b.gif)
Figure 4. Distribution maps of Nigella damascena (both morphs, wild and cultivated) based on national and regional databases. (A) Number of observations adapted from the SIFlore dataset. (B) Collection dates adapted from the SIFlore dataset. (C) Occurrence data adapted from the INPN dataset.
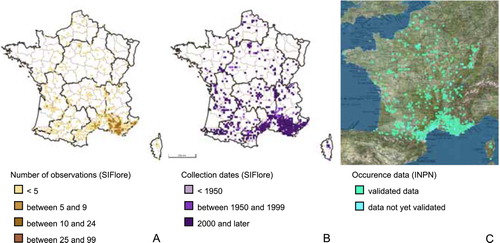
The fact that N. damascena has often been collected next to areas with high human population density could be due to the local presence of many collectors and to the naturalization of populations after escaping from gardens. Whilst this explanation could stand for the Parisian region, we do not think it is valid for the whole French territory, as N. damascena has not been heavily collected from many densely inhabited areas in northern France. Thus, we believe that the distribution of the species at the Atlantic coast, at the Rhône Valley and at the Mediterranean coast represent its true distribution area. The data made available online by SIFlore (Just et al. Citation2015), gathering distributional data for vascular plants in France from herbarium records but also from the literature and data from botanical conservatories and covering the last five centuries (from 1545 to 2014) and by the INPN (Muséum national d’Histoire Naturelle Citation2003–2017) are in accordance with our results, considering that these datasets distinguish neither the [P] from the [T] morph nor wild from cultivated specimens (Figure ).
The morph [T] has been less collected than the [P] morph over the last couple of centuries, suggesting that such populations are less common in the wild than the [P]-morph populations (72/430 = 17%). One-tenth of the collectors of N. damascena collected both floral morphs, and more than half of them found both morphs in the same locality. This suggests that at least 21% (15/72) of the [T] phenotype appears in mixed [P]-[T] populations.
Additionally, the two morphs are not equally distributed in France. Relatively more [T] specimens have been collected in the northern part of France and there is no reason to think that the collectors mostly living or botanizing in the northern half of France were more sensitive to unusual phenotypes. The presence of [T] populations further away from the Mediterranean and the Atlantic coasts could be due to the naturalization of populations in the vicinity of towns and villages after escaping from the gardens. The fact that most cultivated specimens, which are enriched in [T] morphs, were also collected from the northern half of France is consistent with this hypothesis.
A dataset valuable for diachronic evo-devo studies
Museum time-series (set of specimens from the same species collected at different times) are the basic material for floras and conservation studies. Thanks to the information gathered on the collection label, the distribution area of a single species can be traced over time (e.g. Auderset Joye, Castella, and Lachavanne Citation2002). Such diachronic studies can be conducted at the scale of some centuries and do not result from any inference-based analysis. They differ from studies of historical biogeography in which older (thousand- to million-year old) dispersal events are inferred from the distribution areas of the extant taxa and the topology of a phylogenetic tree.
The distribution maps we built are based on the georeferencing of the collection localities of 421 individuals across 200 years. Among this set of individuals, only three could be georeferenced with high precision, meaning the maps presented in Figure mostly show broad localities. However, comparing maps obtained from [P] and [T] specimens can allow us targeting localities where populations of both morphs could currently coexist. Such natural populations would be required for in situ long-term ecological studies.
Ancient DNA (i.e. extracted from relatively old herbarium specimens) has been sequenced to track invasion routes (Matsuhashi et al. Citation2016) or analyze the evolution of the genetic diversity of a plant pathogen (Li et al. Citation2007) through time. In the case of N. damascena, mining our database for old and modern specimens from different origins will make it possible to test whether the molecular basis for the [T] phenotype is identical at the scale of the Mediterranean Basin as a whole, over the last two centuries. This research is currently under way in our team.
Notes on contributors
Catherine Damerval is senior researcher at Centre national de la Recherche Scientifique. Her research deals with evo-devo of the flower in Ranunculales.
Wafa Ben Othman is an agronomist, recently graduated from AgroParisTech.
Domenica Manicacci is an assistant professor at Université Paris-Sud. She is a population geneticist working on different angiosperm models such as maize and Nigella damascena.
Florian Jabbour is an associate professor at the Muséum national d’Histoire Naturelle and a curator at Paris Herbarium. His research focuses on the evolution of plant taxa and plant shapes. He is on the editorial board of Botany Letters.
For this publication, CD, WBO, DM and FJ gathered data, CD and FJ analyzed the results and wrote a first version of the manuscript, and all coauthors contributed to the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental material for this paper is available online at https://doi.org/10.1080/23818107.2017.1422437
TABG_1422437_SuppTable1.xlsx
Download MS Excel (166.2 KB)Acknowledgments
We thank the curators and staff members of B (Robert Vogt and Ulrike Starck), BC (Neus Nualart Dexeus), BEI (Nada Sinno Saoud), BM (Mark Carine and Jacek Wajer), CAI (Hasnaa Hosni), E (Lesley Scott), FI (Lorenzo Cecchi), FR (Georg Zizka and Rainer Doering), G (Cyrille Chatelain), HUJ (Jotham Ziffer-Berger), K (Marie-Hélène Weech), MA (Charo Noya Santos), MEL (Helen Barnes), MPU (Caroline Loup), PAL (Gianniantonio Domina), S (Arne Anderberg), TO (Laura Guglielmone), UPS (Mats Hjertson), W (Ernst Witek), for sending herbarium specimens on loan and/or sending digital images. We thank the staff members of P Herbarium for their technical help and the Institute for Systematics, Evolution and Biodiversity for funding the internship of W. Ben Othman. We also thank two anonymous reviewers for their critical and helpful comments and suggestions.
References
- Auderset Joye, D. , E. Castella , and J. B. Lachavanne . 2002. “Occurrence of Characeae in Switzerland over the Last two Centuries (1800–2000).” Aquatic Botany 72 (3-4 The different uses of herbarium specimens in modern science): 369–385. doi:10.1016/S0304-3770(01)00211-X.
- Clusius, C. 1601. “Melanthium pleno flore.” In Rariorum Plantarum Historia , 207–208. Antwerp: Ex officina Plantiniana Apud Ioannem Moretum.
- Darwin, C. 1897. The Different Forms of Flowers on Plants of the Same Species . New York: D. Appleton.
- Deroin, T. , C. Damerval , M. Le Guilloux , and F. Jabbour . 2015. “Floral Vascular Patterns of the Double-Flowered and Wild-Type Morphs of Nigella damascena L. (Ranunculaceae).” Modern Phytomorphology 7: 13–20.
- Gonçalves, B. , O. Nougué , F. Jabbour , C. Ridel , H. Morin , P. Laufs , D. Manicacci , and C. Damerval . 2013. “An APETALA3 Homolog Controls both Petal Identity and Floral Meristem Patterning in Nigella damascena L. (Ranunculaceae).” The Plant Journal 76 (2): 223–235. doi:10.1111/tpj.12284.
- Heiss, A. G. , and K. Oeggl . 2005. “The Oldest Evidence of Nigella damascena L. (Ranunculaceae) and its Possible Introduction to Central Europe.” Vegetation History and Archaeobotany 14 (4): 562–570. doi:10.1007/s00334-005-0060-4.
- Jabbour, F. , M. Udron , M. Le Guilloux , B. Gonçalves , D. Manicacci , S. Nadot , and C. Damerval . 2015. “Flower Development Schedule and AGAMOUS-like gene Expression Patterns in two Morphs of Nigella damascena (Ranunculaceae) Differing in Floral Architecture.” Botanical Journal of the Linnean Society 178 (4): 608–619. doi:10.1111/boj.12297.
- Just, A. , J. Gourvil , J. Millet , V. Boullet , T. Milon , I. Mandon , and B. Dutrève . 2015. SIFlore, a dataset of Geographical Distribution of Vascular Plants Covering Five Centuries of Knowledge in France: Results of a Collaborative Project Coordinated by the Federation of the National Botanical Conservatories. PhytoKeys 56: 47–60. doi:10.3897/phytokeys.56.5723.
- Le Bras, G. , M. Pignal , M. L. Jeanson , S. Muller , C. Aupic , B. Carré , G. Flament , et al . 2017. “The French Muséum national d’histoire naturelle Vascular Plant Herbarium Collection Dataset.” Scientific Data 4: 170016. doi:10.1038/sdata.2017.16.
- Li, W. , Q. Song , R. H. Brlansky , and J. S. Hartung . 2007. “Genetic Diversity of Citrus Bacterial Canker Pathogens Preserved in Herbarium Specimens.” Proceedings of the National Academy of Sciences 104 (47): 18427–18432. doi:10.1073/pnas.0705590104.
- Matsuhashi, S. , H. Kudoh , M. Maki , M. Cartolano , M. Tsiantis , T. Itagaki , and S. Sakai . 2016. “Invasion History of Cardamine hirsuta in Japan Inferred from Genetic Analyses of Herbarium Specimens and Current Populations.” Biological Invasions 18 (7): 1939–1951. doi:10.1007/s10530-016-1139-9.
- Muséum national d’Histoire naturelle . 2003–2017. “Inventaire National du Patrimoine Naturel.” https://inpn.mnhn.fr
- Renner, S. S. , and A. Rockinger . 2016. “Is Plant Collecting in Germany Coming to an End?” Willdenowia 46 (1): 93–97. doi:10.3372/wi.46.46106.
- Shorthouse, D. P. 2010. “SimpleMappr, an Online Tool to Produce Publication-quality Point Maps.” http://www.simplemappr.net
- Thiers, B. 2017. “Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff, New York Botanical Garden’s Virtual Herbarium.” http://sweetgum.nybg.org/science/ih/
- Toxopeus, H. J. 1927. “Erblichkeitsuntersuchungen an Nigella Damascena L.” Genetica 9: 341–442.10.1007/BF01508296
- Zhang, R. , C. Guo , W. Zhang , P. Wang , L. Li , X. Duan , Q. Du , et al . 2013. “Disruption of the Petal Identity Gene APETALA3-3 is Highly Correlated with Loss of Petals within the Buttercup Family (Ranunculaceae).” Proceedings of the National Academy of Sciences 110 (13): 5074–5079. doi:10.1073/pnas.1219690110.

![Figure 2. Distribution area of Nigella damascena in France over the last two centuries, broken up in 50-year periods. (A–D) Distribution area of the [P] morph. (E–H) Distribution area of the [T] morph.](/cms/asset/5241671e-3e66-4237-85c0-04f4b6a58811/tabg_a_1422437_f0002_b.gif)