ABSTRACT
Plasmalemmasomes (PLSs) are intrusions of the plasmalemma into the cytoplasm and further into the vacuole. A dramatically increased frequency of PLSs was found in mesophyll cells of developing and mature spinach leaves sprayed with Ti-ascorbate as compared to the K-ascorbate and water control. Some details of their early development are presented. The increased frequency of PSs is discussed in the context of eustress, aquaporins and increased water-flux.
Introduction
Plasmalemmasomes (PLSs) are known to be initiated as omega-shaped small engulfings of the plasmalemma (PL) into the cytoplasm. By their growth they reach the tonoplast (T) and push it into the vacuole. From this stage there are two compartments in them, peripherally a narrow symplastic phase between the PL and T, while in the interior there is an apoplastic phase.
Formerly PLSs were suspected to be artifacts, but freeze-fracturing disproved this supposition (Marchant and Moore Citation1973). They were discerned from lomasomes, which are the fusion products of exocytotic vesicles with the PL. These two kinds of formations were termed collectively paramural bodies (Marchant and Robards Citation1968).
In this study we describe the inducibility of PLSs formation, and the changes of their morphology during their developmental progress from the PL to the vacuole.
Materials and methods
Seeds of spinach (Spinacia oleracea cv. Popey) were sown in three plots in the experimental field of Faculty of Horticultural Sciences, Szent István University, Soroksár (Budapest). Six months later the plants were sprayed either with 10 mg/L Ti (titanium)-ascorbate (Titavit, patented by Pais and Fehér Citation1977), or with K-ascorbate of the same ascorbate content, or with deionized water in the different plots, respectively (cca. 0.1 L/m2). Ti can be taken up effectively by plants from the ascorbate complex. Spraying was repeated three days later, then after another three days leaves were sampled for electron microscopy.
Mature leaf pieces were fixed in 4% v/v glutaraldehyde (0.035 M phosphate buffer, pH 7.2) for 2 hours, postfixed in 1% OsO4 w/v for 2 hours, then contrasted in 2% uranyl acetate for 30 min. Developing leaf pieces (0.8–1 cm blade length) were fixed in 2% v/v glutaraldehyde (0.05 M cacodylate buffer pH 7.4 containing 10 mM CaCl2), washed in the same buffer with CaCl2, postfixed in 2% w/v OsO4 plus 0.8% K3Fe(CN)6 for 2 hours according to Hepler (Citation1981), then contrasted as the mature leaf pieces. All samples were dehydrated in an ethanol series and propylene oxide, then embedded in Durcupan ACM (Fluka). Sections made with a Reichert-Jung Ultracut E ultramicrotome were stained on grids (Pb-citrate), then examined in a Tesla BS 500 electron microscope at 60 kV. Images taken on Forte Scifort films were printed then digitized in an EPSON Perfection 1650 scanner.
Results
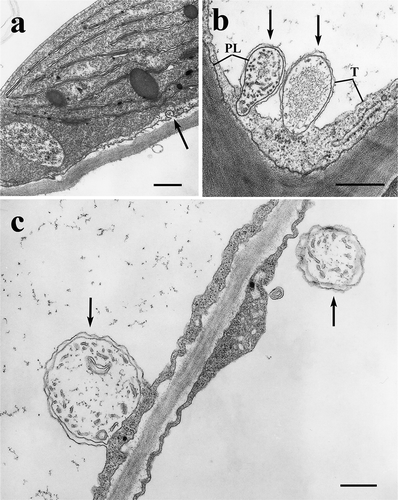
In developing leaves the osmium+ferricyanide fixation increased the contrast of the plasmalemma (), which showed frequent invaginations after Ti-ascorbate treatment ()). These were narrow and curved, their tip seemed to be in contact with their base, or being fused with it, giving rise to concentric membrane pairs along the PL. In the K-ascorbate and water controls such invaginations were rare or absent, respectively (). Apart from these there appear dense deposits within or on the PL in the treated leaves.
Figure 1. Plasmalemma (PL) and early plasmalemmasomes (PLSs) in developing spinach leaves. a: Ti-ascorbate treatment, b: K-ascorbate treatment, c: deionized water treatment. Arrows point to PLSs. CW = cell wall; P = chloroplast; Bars equal 0.5 µm.
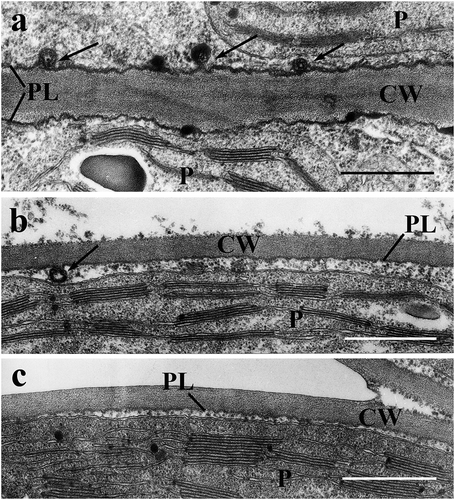
In mature leaf mesophyll cells the Ti-ascorbate treatment definitely increased the frequency of formations easily identifyable as PLSs, which were much less abundant or absent in the K-ascorbate and water controls. Different developmental stages were recorded from initiation ()) through intruding ()), then detaching into the vacuole ()). On the periphery (between T and PL) there is a clear cytoplasmic phase, in their apoplastic interior there are tubules and vesicles. Assuming that these formations develop from further invaginations of the PL, their lumina may belong to the symplastic (cytoplasmic) phase. The apoplastic part sometimes contain fine fibrillar precipitate ()).
Discussion
The increased contrast of PL after osmium+ferricyanide fixation tells the origin of the membranous formations being in contact with it. As these membranes are also dense, they have to originate from the PL, rather than from other intracellular membranes. The dense membrane deposits in PL are of unknown nature. Similar formations appeared in Arabidopsis after cold treatment (Ristic and Ashworth Citation1993), but this is not likely in our case, as the spinach leaves were sampled in August.
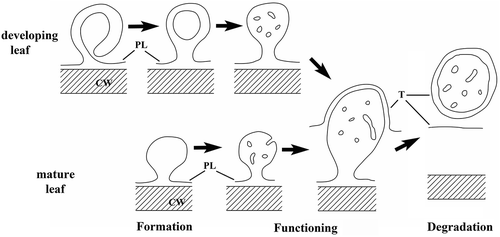
The way of formation of early PLSs (in developing leaves) has not been demonstrated hitherto (to our best knowledge), and we summarize it in . In mature leaves we were not able to recognize these precursor forms, here the simpler omega-shaped intrusions occurred. The ontogenetic connection between the different forms of PLSs from the PL to the vacuole was proven by immunolabeling of membrane-bound glycoproteins (Herman and Lamb Citation1992). On this basis we regard the PLSs in as developmental stages, i.e. initiation, growth, then sequestering (presumably digestion) in the vacuole ()).
The function of the PLSs had been elusive until the assembly of specific aquaporins (PIP1) was discovered in them (Robinson et al. Citation1996). As another class of aquaporins (TIPs) was localized in the T (rewieved by Kaldenhoff and Fischer Citation2006), the close spatial relationship of PL and T in the mature PLSs may facilitate the water transport between the apoplast and the vacuole.
The inducibility of PLS formation was reported in different objects. In Chara cells the basic pH of the medium increased the frequency of charasomes analogous to PLSs (Lucas, Keifer, and Pesacreta Citation1986). In Helianthus stem a few days of water-logging induced PLS formation (Ni et al. Citation2019). In Rubia suspension culture cells different fungal elicitors enhanced the occurrence of PLSs (Bóka, Jakab, and Király Citation2002). In our experiments reported here Ti-ascorbate had a similar effect in spinach leaves. Considering that the controls (K-ascorbate and water) had very low PLS frequencies, it is the Ti which can be responsible for the increase. Ti was found to have beneficial effect on various developmental and physiological parameters of crop plants (Pais, Fehér, and Farkas Citation1979; Ram, Verloo, and Cottenie Citation1983).
However, not only Ti has beneficial effect, but also other metal ions (Cd, Pb, Ni), or even DCMU, if administered in a very low (sub-micromolar) concentration (Nyitrai et al. Citation2003). The more toxic the agent, the lower the stimulative concentration is. These treatments caused stimulation (eustress) in bean and barley seedlings, and a delay or reversion of senescence in the ageing leaves of these species by activating the phosphoinositide – MAPK signaling pathway (Nyitrai et al. Citation2007, Citation2009; Kovács, Nyitrai, and Keresztes Citation2010). The positive effect of Ti-treatment on crop yield (Kalinowski and Wadas Citation2017) fits well into the scheme of eustress, i.e. the enhanced metabolism. This necessitates an increased ion uptake (Maróti, Pais, and Bognár Citation1984) accompanied with higher water-influx, and this connects eustress to more aquaporins in more PLSs. To induce eustress Ti is a very suitable agent, as it is not toxic, so it does not cause environmental problems.
Acknowledgments
We are indebted to Dr. Klára Papp for providing the plant material. We thank Mrs. Sarolta Pálfia for her excellent microtechnical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Áron Keresztes
Áron Keresztes (corresponding author) is plant cell biologist working at the Eötvös Loránd University. Contribution: general concept and research question, organization of experimental work, interpretation of results, manuscript preparation.
Károly Bóka
Károly Bóka is a plant cell biologist working at the Eötvös Loránd University. Contribution: interpretation of results and manuscript preparation.
References
- Bóka, K., J. Jakab, and I. Király. 2002. “Comparison of the Effect of Different Fungal Elicitors on Rubia tinctorum L. Suspension Culture.” Biologia Plantarum 45: 281–290. doi:10.1023/A:1015113226897.
- Hepler, P. K. 1981. “The Structure of Endoplasmic Reticulum Revealed by Osmium Tetroxid – Ootassium Ferricyanide Staining.” European Journal of Cell Biology 26: 102–110.
- Herman, E. M., and C. J. Lamb. 1992. “Arabinogalactan-rich Glycoproteins are Localized on the Cell Surface and in Intravacuolar Multivesicular Bodies.” Plant Physiology 98: 264–272. doi:10.1104/pp.98.1.264.
- Kaldenhoff, R., and M. Fischer. 2006. “Functional Aquaporin Diversity in Plants.” Biochimica et Biophysica Acta 1758: 1134–1141. doi:10.1016/j.bbamem.2006.03.012.
- Kalinowski, K., and W. Wadas. 2017. “Effect of Tytanit on the Yield and Yield Components of Very Early-maturing Potato Cultivars.” Journal of Central European Agriculture 18: 441–459. doi:10.5513/JCEA01/18.2.1917.
- Kovács, E., P. Nyitrai, and Á. Keresztes. 2010. “How Does a Little Stress Stimulate a Plant?” Plant Signaling and Behaviour 5: 354–358. doi:10.4161/psb.5.4.10870.
- Lucas, W. J., D. W. Keifer, and T. C. Pesacreta. 1986. “Influence of Culture Medium pH on Charasome Development and Chloride Transport in Chara corallina.” Protoplasma 130: 5–11. doi:10.1007/BF01283326.
- Marchant, R., and R. T. Moore. 1973. “Lomasomes and Plasmalemmasomes in Fungi.” Protoplasma 76: 235–247. doi:10.1007/BF01280700.
- Marchant, R., and A. W. Robards. 1968. “Membrane Systems Associated with the Plasmalemma of Plant Cells.” Annals of Botany 32: 457–471. doi:10.1093/oxfordjournals.aob.a084221.
- Maróti, M., I. Pais, and J. Bognár. 1984. “The Role of Titanium in Plant Life V. The Effect of Titanium on the Growth of Tobacco Callus.” Acta Agronomica Academiae Scientiarum Hungaricae 33: 315–322.
- Ni, X. L., M. I. Gui, L. L. Tan, Q. Zhu, W. Z. Liu, and C. X. Li. 2019. “Programmed Cell Death and Aerenchyma Formation in Water-logged Sunflower Stems and Its Promotion by Ethylene and ROS.” Frontiers in Plant Science 9: 1928. doi:10.3389/fpls.2018.01928.
- Nyitrai, P., K. Bóka, L. Gáspár, É. Sárvári, K. Lenti, and Á. Keresztes. 2003. “Characterization of the Stimulating Effect of Low-dose Stressors in Maize and Bean Seedlings.” Journal of Plant Physiology 160: 1175–1183. doi:10.1078/0176-1617-00770.
- Nyitrai, P., P. Czövek, M. Óvári, and Á. Keresztes. 2009. “Signaling Mechanism Mediating the Anti-senescence Effect of Low-concentration Chemical Stressors Sprayed onto Bean Seedlings.” Environmental and Experimental Botany 66: 501–506. doi:10.1016/j.envexpbot.2009.04.009.
- Nyitrai, P., M. Mayer, M. Óvári, and Á. Keresztes. 2007. “Involvement of the Phophoinositide Signaling Pathway in the Anti-senescence Effect of Low-concentration Stressors on Detached Barley Leaves.” Plant Biology 9: 420–426. doi:10.1055/s-2006-924761.
- Pais, I., and M. Fehér. 1977. Hungarian Patent A 01 N5/100.
- Pais, I., M. Fehér, and E. Farkas. 1979. “Role of Titanium in the Life of Plants. I.” Acta Agronomica Academiae Scientiarum Hungaricae 28: 378–383.
- Ram, N., M. Verloo, and A. Cottenie. 1983. “Response of Bean to Foliar Spray of Titanium.” Plant and Soil 73: 285–290. doi:10.1007/BF02197724.
- Ristic, Z., and E. N. Ashworth. 1993. “Changes in Leaf Ultrastructure and Carbohydrates in Arabidopsis thaliana L. (heyn) Cv. Columbia during Rapid Cold Acclimation.” Protoplasma 172: 111–123. doi:10.1007/BF01379368.
- Robinson, D. G., H. Sieber, W. Kammerloher, and A. R. Schaeffner. 1996. “PIP1 Aquaporins are Concentrated in Plasmalemmasomes of Arabidopsis thaliana Mesophyll.” Plant Physiology 111: 645–649. doi:10.1104/pp.111.2.645.