ABSTRACT
The known fossil-species of upper Carboniferous (Pennsylvanian) arborescent lycopod leafy shoots are re-examined. The leaf characters used in species characterisation include size (length and breadth), angle of departure from the stems and the overall shape (straight, curved or S-shaped). Sometimes leafy shoots can be directly related to known species of stems that are defined on their surface features of leaf cushions as in Lepidodendron, Lepidophloios and Ulodendron, or directly on the stem surface as in Bothrodendron. They are also assessed in terms of the determinate growth pattern of the plants. A key is given to identify the fossil-species of leafy shoots described here.
Introduction
Arborescent lycopods were among the most abundant plants of the Palaeozoic coal swamps () having spread over large areas of tropical land during the late Mississippian (Serpukhovian) to Cisuralian (early Permian), and were ecological keystone taxa of this important biome (DiMichele and Phillips Citation1995). A combination of this abundance and a favourable taphonomic setting has meant that adpressions of these plants are widespread in rocks of this age. However, because they shed organs during life and were disarticulated during the fossilisation process, different parts of the plant are assigned to different fossil-taxa (Cleal and Thomas Citation2010, Citation2021). This has sometimes caused confusion, especially when the fossil record of these plants is being used for diversity, palaeoecological or floristic studies (as discussed by Cleal et al. Citation2021).
Figure 1. Reconstruction of arborescent lycopsids of the Pennsylvanian-age palaeotropical coal swamps of Euramerica. Artist Annette Townsend.

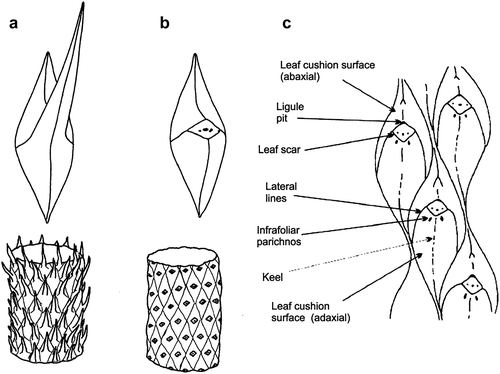
Particularly difficult has been the taxonomy of their leafy shoots. The stems are normally classified using features of the leaf cushions, but in the shoots, these cushions tend to be obscured by the leaf laminae (). There have been a number of earlier taxonomic accounts of these leafy shoots (Fischer Citation1904; Nĕmejc Citation1947; Crookall Citation1964; Chaloner Citation1967) but there has been little consensus as to how many taxa can be recognised and how to determine the variability within them. It is, therefore, time to reappraise the taxonomy of these fossils, and present here a revised classification and an identification key based on characters that should allow them to be reliably and consistently recorded.
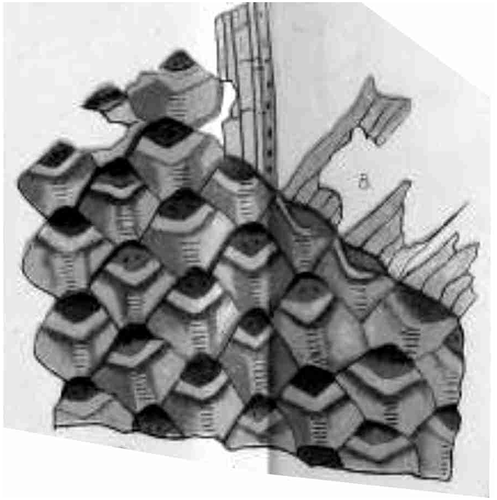
Figure 2. Lepidodendron stems. (a) Stems with leaves still attached; (b) Stems showing scars after leaves have been abscised; (c) Main characters of leaf cushions and scars used to identify stems. Adapted from Cleal and Thomas (Citation1994, Citation2019), based on drawings by D. Spillards.
Growth and taphonomy
Palaeozoic arborescent lycopods had a main vertical trunk borne on a stigmarian rhizomorph base that provided a platform to facilitate growth in unstable, swampy substrates (Thomas and Seyfullah Citation2015a; DiMichele et al. Citation2022). The trunk, which was at least partly covered by elongate linear leaves (named Cyperites Lindley and Hutton Citation1831-1831b), could be at least 35 m tall (Thomas and Watson Citation1976; Thomas Citation1978).
Some of these lycopods retained the elongate leaves on their stem throughout their life (Thomas Citation1967b) and occasionally the lower part of the trunk has been found with leaves still attached (e.g. Thomas Citation1970; Leary and Thomas Citation1989). In most fossils of such stems, however, the leaves are no longer present because they have dried and withered (Thomas Citation1978; Thomas and Meyen Citation1984a, Citation1984b) leaving a mark on the cushion where the leaf blade entered the matrix of the counterpart called a false leaf scar (Chaloner Citation1967).
In most Pennsylvanian species, however, the leaves were actively abscised as the trunk grew (Thomas and Cleal Citation1999) leaving the swollen clasping bases of the leaves (leaf cushions), each with a scar where the leaf lamina had been detached. A real leaf scar resulting from leaf abscission can be distinguished from a false scar by the presence of three foliar prints marking where the vascular trace and two parichnos entered the leaf, and a different surface-cellular arrangement to that on the surrounding leaf cushion (Thomas Citation1978; Thomas and Cleal Citation1999). The leaf cushions remained photosynthetic and so were presumably green (Thomas Citation1966, Citation1974; Thomas and Cleal Citation2018). With further growth of the plant, however, the outer tissue of the trunk with the leaf cushions was also sloughed off exposing the underlying non-photosynthetic periderm (remains of the stems where the outer tissue has been shed are referred to the fossil-genus Knorria Sternberg Citation1825). Sloughed-off portions of the outer tissue with the characteristic leaf cushions are the most abundant remains of these stems.
The anatomy of the trunk (Walton Citation1935; Andrews and Murdy Citation1958; Eggert Citation1961) and the exponential relationship between stem diameter and leaf length (Chaloner and Meyer-Berthaud, Citation1983) indicates these plants had determinate growth. On reaching maturity the plants produced sporangia, which could either be attached singly to the trunk (e.g. Omphalophloios White Citation1898 ‒ Bek et al. Citation2015; Wagner and Álvarez-Vázquez Citation2015) or borne in strobili. The strobili of Sigillaria-plants were attached laterally to the trunk but in most other genera they were borne terminally on slender leafy shoots that formed a crown at the top of the plant (e.g. Opluštil Citation2010). These shoots are the subject of this paper.
Taxonomic and nomenclatural background
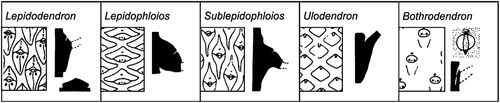
Stem adpressions of the plants that produced the leafy shoots are assigned to the fossil-genera Lepidodendron Sternberg Citation1820, Lepidophloios Sternberg Citation1825, Sublepidophloios Sterzel Citation1907, Ulodendron Lindley and Hutton Citation1831 and Bothrodendron Lindley and Hutton Citation1833a mainly on leaf cushion features (). However, Kidston (Citation1886) showed that the species taxonomy of these stem adpressions can be extremely difficult because of the variation in cushion size and shape. Kidston believed that the smaller and larger cushions were from the young and older stems, but we now know that this variation in size was an expression of the position of the cushions on the plant (Eggert Citation1961).
Figure 3. Main fossil-genera of stems recognised in the Pennsylvanian arborescent lycopods Adapted from Cleal and Thomas (Citation1994, Citation2019).
There are about 20 named species of leafy lycopod shoots, which is far fewer than the several hundred stem species, so there must be other species of leafy shoots waiting to be identified. There is also the problem of how to relate the leafy shoots to the larger leaf cushion-bearing stems because there are only a few examples known where continuity has been demonstrated between the two (e.g. Nĕmejc Citation1947; Chaloner and Meyer-Berthaud Citation1983; Thomas et al. Citation2010; Opluštil Citation2010).
We have not attempted a detailed analysis of the distribution of the fossil-species of lycopod leafy shoots. Rather, we have tried to develop the basis of a taxonomy that will allow these fossils to be accurately documented. Where we have included stratigraphic details of species, it has been where we are confident about the records (including of the relevant nomenclatural types), but this should in no way be regarded as a comprehensive documentation of their distribution.
Descriptions of species
These descriptions deal mainly with the morphological features. Epidermal features preserved on cuticles are discussed by Thomas (Citation1966, Citation1967a, Citation1967b, Citation1968, Citation1970, Citation1974, Citation1977). Where possible, we have illustrated photographs of the relevant type specimens and, in some cases also the original published figure.
Genus: Lepidodendron Sternberg Citation1820
Remarks
This generic name was originally given to remains of stems covered with leaf cushions, each with an abscission scar, but it has also been used for the leafy shoots that formed the crown of the plants. Brongniart (Citation1822, p. 209) referred any fossil shoots bearing simple, linear leaves arranged helically or in two rows to the genus Lycopodites. This was based on the earlier but invalidly published Lycopodiolithes Schlotheim Citation1820, but which Brongniart re-spelled to avoid the implication that the genus only included lycopod shoots; using a similar logic Sternberg (Citation1825) subsequently further changed the spelling to Lycopodiolites. Brongniart divided Lycopodites into four informal sections, the first of which included shoots produced by plants with Sagenaria Brongniart Citation1822 (≡ Lepidodendron Sternberg) stems. However, he also specifically stated that the most typical species of Lycopodites were those in the second of the sections (Lycopodites taxiformis Brongniart Citation1822, Lycopodites piniformis Schlotheim ex Brongniart Citation1822) which are now regarded as coniferous shoots (Florin Citation1938‒1945). Because of this confusion, the generic name has now been formally conserved as Lycopodites Lindley and Hutton Citation1833b and is restricted to the remains of herbaceous lycopods (Pal and Gosh Citation1990).
Álvarez-Vázquez and Wagner (Citation2014) and Álvarez-Vászuez et al. (Citation2018) also suggested that the leafy shoots and shoots lacking true leaf scars should be assigned to a different fossil-genus, which they called Bergeria Sternberg Citation1838. As pointed out by Thomas and Cleal (Citation2020), however, the lectotype species of Bergeria (B. acuta Presl in Sternberg Citation1838 ‒ designated by Andrews Citation1955) has leaf cushions very similar in shape to Lepidodendron except that they lack true leaf scars. It is known that Lepidodendron stems with rhomboidal cushions retained their leaves for much of their life (e.g. Thomas Citation1978; Leary and Thomas Citation1989), only shedding them when the terminal crown was produced. Most adpressions of Lepidodendron represent portions of the periderm that had been sloughed-off from the trunk and after the leaves had been abscised producing true leaf scars. It seems likely, therefore, that the leafy shoots that have been assigned to Bergeria are merely the more distal parts of a Lepidodendron branching system where the leaves have not been abscised. Whilst different biological species may have had different proportions of the stem that abscised their leaves, possibly related to the stature of the species (species that grew larger may have sloughed-off more periderm and so produced more adpressions of leaf cushions with true leaf scars), there seems to be no sharp delineation between those leaf cushions that would be assigned to Bergeria and those that would be assigned to Lepidodendron. The distinction is, therefore, of doubtful taxonomic merit at least at the rank of genus (Thomas et al. Citation2019; Thomas and Cleal Citation2020; DiMichele and Bateman Citation2020).
Lepidodendron dawsonii Bell Citation1838
Figure 4. (a) Lepidodendron dawsonii Bell, Geological Survey of Canada, Specimen 3250 (holotype), Dominion No. 16 Colliery, Sydney Coalfield (Cape Breton, Canada); roof of the Phalen Seam, Morien Group (Asturian); original illustration by Bell (Citation1938, pl. 99, fig. 4). (b, c), Lepidodendron pictoense Dawson, Redpath Museum, McGill University, Specimen 3478 (lectotype); Sydney Coalfield, Nova Scotia (Canada); Morien Group (Asturian). (b), Original illustration by Dawson (Citation1866, fig. 37a). (c), Photograph of specimen (reproduced with permission of the Redpath Museum, McGill University).
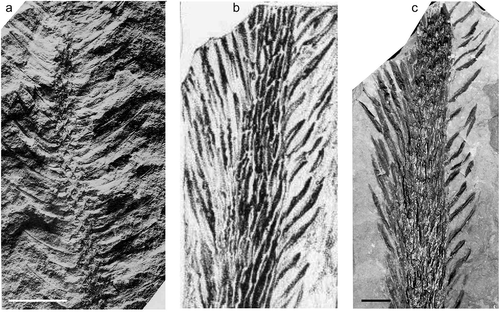
Citation1938 Lepidodendron dawsoni Bell, p. 94, pl. 98, ; pl. 99, .
Citation1980 Lepidodendron dawsoni Bell; Zodrow and McCandlish, p. 80, pl. 119.
Holotype
Geological Survey of Canada, Ottawa, Specimen No. 3250 (Bell Citation1938, pl. 99, ); Provenance, Dominion No. 16 Colliery, Sydney Coalfield (Cape Breton, Canada); roof of the Phalen Seam, Morien Group (Asturian).
Description
Leaf cushions of low relief, 4‒5 times longer than wide, with leaf scars just above the middle of the cushion and up to ¾ its width. Leafy shoots, 3‒30 mm wide with acutely pointed leaves up to 17 mm long and 0.75 mm wide, departing from the leaf cushions at right angles before obliquely arching upwards.
Remarks
This species is mainly known from leafy shoots and slender stems up to ca. 10 mm wide with leaf cushions. The most detailed discussion of this species since the publication of the protologue has been by Zodrow et al. (Citation2012), who included details of the cuticles. The only other species with leaves attached at right angles to the cushion is L. ophiurus Brongniart, but the latter has more curved leaves that become approximately parallel to the shoot in their distal parts.
Occurrence
This species has so far only been recorded from the Morien Group (between Emery and Point Aconi seams ‒ Asturian) of the Sydney Coalfield, Nova Scotia, Canada.
Lepidodendron dilatatum Lindley and Hutton Citation1831
Figure 5. Lepidodendron dilatatum Lindley and Hutton. (a, b), Great North Museum, Specimen No. NEWHM G02.16 (holotype); Felling Colliery, Gateshead, Tyne and Wear (UK); roof of the L Main Seam (Duckmantian); (a), original illustration given with protologue (Lindley and Hutton Citation1831); (b), photograph, by Sylvia Humphrey. (c), National Museum Wales, Specimen NMW Appleton BB0076A; Brymbo Opencast near Wrexham, Denbigh Coalfield, north Wales, UK; Middle Coal Measures (Duckmantian) (photo by P. Appleton). (d), Drawing of leafy shoot by Deborah Spillards (from Cleal and Thomas, Citation1994). All scale bars = 10 mm.
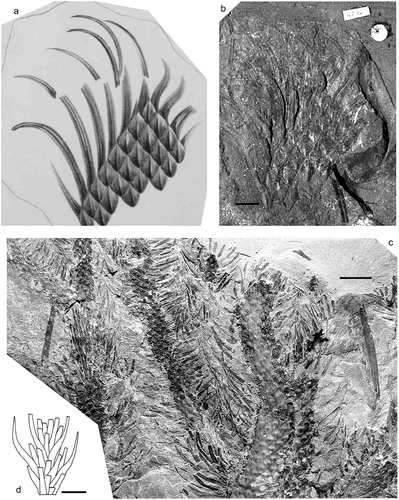
Citation1831 Lepidodendron dilatatum Lindley and Hutton, pl. 7 .
Citation1838 Bergeria acuta Presl in Sternberg, pl. 48, .
Citation1848 Lycopodites dilatatus (Lindley and Hutton) Göppert ex Bronn, p. 681.
Citation1854 Lepidodendron haidingeri Ettingshausen, p. 55, pls, 22, 23.
Citation1854 Lepidodendron vestitum Lesquereux, p. 428.
Citation1858 Lepidodendron vestitum Lesquereux, p. 874, pl. 16, .
Citation1880 Lepidodendron scutatum Lesquereux, p. 369 (1879, pl. 63, ).
Citation1880 Lepidodendron lanceolatum Lesquereux, p. 369 (1879, pl. 63, ).
Citation1911 Lepidodendron acutum (Presl in Sternberg) Kidston, p. 146.
Citation1947 Lepidodendron simile Kidston; Němejc (non Kidston), p. 68, pl. 1, , .
Citation2014 Bergeria dilatata (Lindley and Hutton) Álvarez-Vázquez and Wagner, p. 201, .
Citation2018 Bergeria dilatatum (Lindley and Hutton) Álvarez-Vázquez et al., p. 10, .
Holotype
Great North Museum, Newcastle-upon-Tyne, Hutton Collection, Specimen No. NEWHM G02.16; Provenance, Felling Colliery, Gateshead, Tyne and Wear (UK); roof of the Low Main Seam (Duckmantian). Figured photographically here in (see also Álvarez-Vazquez et al. Citation2018, ).
Description
Leafy stems with elongated cushions with acute upper angles, blunt pointed bases and gently rounded sides. The laminae depart at less than 45° to the stem axis and are broader than 3 mm at the base; the leaves are S-shaped at their base and then curve upwards, up to 20‒70 mm long.
Remarks
Álvarez-Vázquez and Wagner (Citation2014) and Álvarez-Vázquez et al. (Citation2018) have provided a detailed taxonomic treatment of this species (under the name Bergeria dilatatum). They in particular documented a range of well-preserved leafy shoots from Canada and Spain, which provide an excellent overview of the variation within the species. However, in our view, their synonymy has tended to lump together too wide a range of shoots, which using the classification suggested here would be assigned to various other fossil-species, notably Lepidodendron lycopodiodes, Lepidodendron ophiurus, Ulodendron majus and Ulodendron landsburgii. Reasons for this are given under the remarks on those species.
Nĕmejc (Citation1947) described and figured examples of this species as Lepidodendron simile Kidston (see also Opluštil et al. Citation2009b, pl. 1) but the status of that name is doubtful. It was first used as a nomen nudum in Jongmans (Citation1909). Kidston (Citation1911) subsequently provided an ambiguous diagnosis, merely stating that it differed from L. lycopodioides. Although no type was illustrated or described, Kidston made specific reference to the specimens figured by Brongniart (Citation1837, pl. 14) as Lepidodendron elegans Sternberg. The Brongniart (Citation1837, p. 47) text for L. elegans provided what might be regarded as a validating diagnosis (“… un autre rameau qui porte des feuilles implantées presque perpendiculairement sur l’axe, coudées et renflées toutes à la même distance de cet axe”) but both this description and the specimens illustrated by Brongniart differ from L. simile as interpreted and illustrated by Nĕmejc (Citation1947); the latter consists of shoots with S-shaped leaves and compares closely with the type of L. dilatatum. In view of this ambiguity as to its type, diagnosis and circumscription, we regard L. simile as a taxonomically unusable name.
Nĕmejc (Citation1947) compared his “L. simile” with the types of L. acutum (≡ Bergeria acuta), the only difference being that the leaf cushions of the latter were larger and lacked attached leaves. Nĕmejc (Citation1947) also pointed out that Lepidodendron haidingeri Ettingshausen, Citation1854 seems to be intermediate between the two species: it has smaller but very similar-shaped leaf cushions to L. acutum, and leaves that are very similar in shape but a little smaller to those of L. dilatatum. Although Nĕmejc (Citation1947) did not combine these species, we suggest that L. acutum, L. haidingeri and L. dilatatum (i.e. Nĕmejc’s L. simile) represent progressively more distal branches from the same biological species.
Nĕmejc (Citation1947) regarded his “L. simile” as conspecific with upper Mississippian leafy shoots from northern France figured by Bureau (Citation1913, pl. 30 bis, ; pls 32‒34) as Lepidodendron lycopodioides Sternberg. However, these shoots have more linear leaves attached at a more acute angle especially in the slightly wider stems (Strullu-Derrien et al. Citation2021). We have yet to see any shoots older than Pennsylvanian that could be assigned to L. dilatatum.
Thomas et al. (Citation2019) synonymised L. dilatatum with Lepidodendron vestitum Lesquereux, Lepidodendron scutatum Lesquereux, and Lepidodendron lanceolatum Lesquereux. Kidston (Citation1891) referred L. dilatatum to Lepidodendron ophiurus (Brongniart) Brongniart, Citation1828 but Crookall (Citation1964) argued that the leaf cushions of the former are much larger. The L. ophiurus leaves are also attached at a more acute angle to the cushions than in L. dilatatum.
Occurrence
L. dilatatum is one of the commonest species of leafy lycopod shoots having been recorded (often as L. acutum) from all the major Euramerican coalfields and from the Langsettian to the Asturian (Crookall Citation1964; Thomas Citation2007; Thomas and Tenchov Citation2004; Álvarez-Vásquez and Wagner Citation2014). The type of L. acutum was from Nýřany Member (Asturian) of Bohemia, Czech Republic, and the type of L. haidingeri was from the Radnice Member (upper Duckmantian) of the same coal-basin. In Pennsylvania it is reported from Brown’s Colliery, Pittston; Archibald Mine B & C vein, Wilkes-Barre (Asturian); and Wanamie, near Wilkes-Barre (Thomas et al. Citation2019); also from the Northern Anthracite Basin (Asturian) as L. lanceolatum (Olesksyshyn Citation1982).
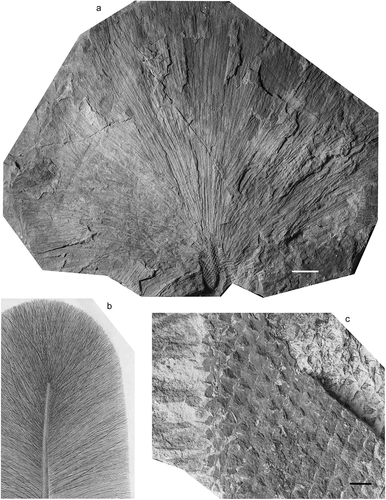
Lepidodendron latifolium Lesquereux Citation1880
Figure 6. Lepidodendron latifolium Lesquereux; Botanical Museum, Harvard University, Specimen No. HU8200; Oliphant coal mines, Pennsylvania (USA); stratigraphical horizon not recorded; original illustration given with protologue (Lesquereux, 1880).
1880 Lepidodendron latifolium Lesquereux, p. 370 (1879, pl. 63, ).
Holotype
Botanical Museum, Harvard University, Cambridge MA (USA), Specimen No. HU8200 (; Lendemer Citation2002); Provenance, coal mines at Oliphant, Pennsylvania (USA); stratigraphical horizon not recorded. We have been unable to obtain a photographic image of the specimen and so we have reproduced the original illustration given with the protologue.
Description
Stems with clearly marked, vaulted, isodiametric-rhomboidal leaf cushions, with a prominent leaf scar in the upper part and transverse wrinkles in the lower part. When attached, long leaves are at least 7 mm broad.
Remarks
There are few records of this extremely rare but distinctive species; other than the protologue, it has only been documented by Noé (Citation1925, pl. 7, ; pl. 8, ) and Gastaldo (Citation1977, ) (there were also equivocal records from Indiana by Canright, Citation1959 and Wood, Citation1968). Although based on only limited characters, we know of no other species that it could be synonymised with. In terms of leaf length, a close comparison can be made with L. longifolium, but the leaves are much broader on L. latifolium.
A single specimen from the Ruhr Coalfield figured by von Röhl (Citation1868, pl. 11, ) as Lepidodendron dichotomum Sternberg has similar large leaves and isodiametric-rhomboidal leaf cushions.
Occurrence
This species is mainly known from Oliphant, Pennsylvania (USA) and from Mazon Creek and the Herrin (No. 6) Coal at Carterville, Illinois (USA). There is also a possible record from the Ruhr Coalfield (Germany),
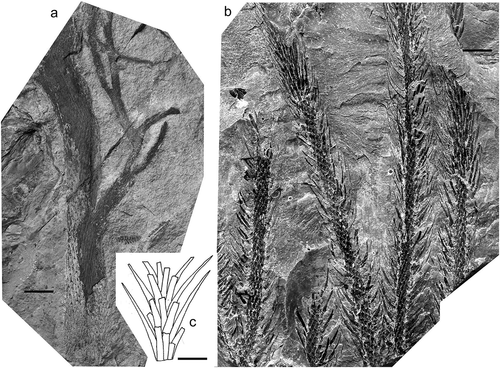
Lepidodendron longifolium Brongniart ex Lindley and Hutton Citation1835
Figure 7. Lepidodendron longifolium Brongniart ex Lindley and Hutton, Národní muzeum (Prague); all specimens from Whetstone Horizon (Duckmantian). (a), Specimen E 7647 (holotype); Svinná, Czech Republic (photo by Lenka Váchová); scale bar = 30 mm. (b), Illustration of the holotype from Sternberg (Citation1820). (c) 1820, close-up of wider stem showing leaf cushions and attached leaves; Chomle, Czech Republic (photo by S. Opluštil; figured by Němejc, Citation1934, pl. 2, fig. 3); scale bar = 10 mm.
1820 Lepidodendron dichotomum, var., Sternberg, pl.3.
Citation1828 Lepidodendron longifolium Brongniart, p. 85 (nom. illegit.).
Citation1835 Lepidodendron longifolium Brongniart ex Lindley and Hutton, p. 37, pl. 161.
1848 Lycopodites longifolius (Brongniart) Göppert in Bronn, p. 682.
Citation1870 Lepidodendron morrisianum Lesquereux, p. 430, pl. 22, .
Citation1880 Lepidodendron morrisianum Lesquereux; Lesquereux, p. 370.
1880 Lepidodendron longifolium Brongniart; Lesquereux, p. 373.
Citation1894 Lepidodendron longifolium Brongniart; Kidston, p. 599, pl. 1, .
Citation1947 Lepidodendron longifolium Brongniart; Nĕmejc, p. 55.
Holotype
Brongniart (Citation1828) renamed one of the syntypes of L. dichotomum Sternberg as a new species, L. longifolium (). The specimen was recorded as originating from Svinná (West and Central Bohemia Coalfield, Czech Republic), presumably from the upper Duckmantian Whetstone Horizon ash band in the Radnice Coal Seam, lower Kladno Formation.
Description
The leaves in L. longifolium are extremely narrow and very long, reaching c. 500 mm in length. Leaf cushions fusiform with a distinct median keel.
Remarks
Although Brongniart (Citation1828, p. 85) first used this species epithet he failed to provide a diagnosis. The earliest legitimate use was by Lindley and Hutton (Citation1835) who made a direct reference to Brongniart (Citation1828 ‒ and so also to the Sternberg type) and provided a diagnosis, albeit rather brief. Unger (Citation1850) doubted the validity of L. longifolium and included it in his “Species dubius nudum descriptae”, but Kidston (Citation1894) and Jongmans (Citation1929) accepted it as a distinct species; Kidston also figured two specimens of his own from South Wales. The main distinguishing character is that the leaves are extremely narrow and very long, reaching 380 mm in Lindley and Hutton’s specimen and 500 mm in Sternberg’s specimen.
The most extensive recent treatment of the species has been by Opluštil et al. (Citation2007, Citation2009a, Citation2014, Citation2016) and Libertín et al. (Citation2009) based on newly collected material from the Whetstone Horizon ash band in the West and Central Bohemia Coalfield, including some stems with the distinctive, long leaves still attached. They found that the leaf cushions lacked infrafoliar parichnos and bore strobili with Cappasporites microspores, and may have been produced by relatively small arborescent lycopods only some 4 m tall.
Lesquereux (Citation1866) based Lepidodendron morrisianum on a single specimen with indistinct leaf cushions, and leaves at least 300 mm long and 5½ mm broad with a central vein. On the length of the leaves and the lack of other distinguishing features, it is included here as a synonym of L. longifolium.
Occurrence
Svinná, Štilec and Ovčin, West and Central Bohemia Coalfield (Czech Republic), upper Duckmantian Radnice Coal Seam, lower Kladno Formation; Brown colliery, E Vein, Pittston, Pennsylvania (USA) (Lesquereux Citation1880); Ebbw Vale, Rhondda Cynon Taff, Wales (UK), South Wales Coal Measures Group (Langsettian to lower Bolsovian).
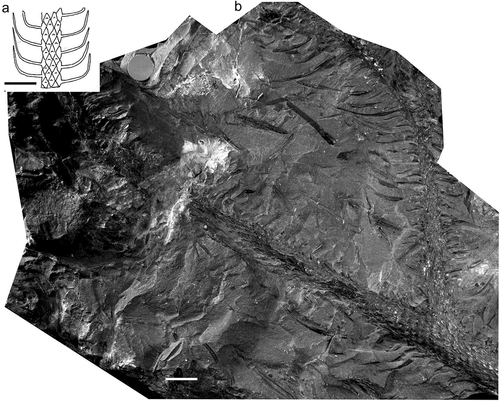
Lepidodendron lycopodioides Sternberg 1821
Figure 8. Lepidodendron lycopodioides Sternberg. (a), Národní muzeum (Prague), Specimen E 4743 (lectotype); Svinná, Czech Republic; Whetstone Horizon (Duckmantian) (photo by Lenka Váchová). (b), National Museum Wales, Specimen NMW 2013.43 G.108; Brymbo Opencast near Wrexham, Denbigh Coalfield, north Wales, UK (photo by P. Appleton); Middle Coal Measures (Duckmantian). (c), Drawing of leafy shoot by Deborah Spillards (from Cleal and Thomas, Citation1994). All scale bars = 10 mm.
Citation1821 Lepidodendron lycopodioides Sternberg, p. 26, pl. 16, .
1825 Lycopodiolithes elegans Sternberg, p. viii (homotypic synonym).
Citation1828 Lepidodendron elegans (Sternberg) Brongniart, p. 85.
Citation1831 Lepidodendron sternbergi Brongniart; Lindley and Hutton (non Brongniart), p. 15, pl. 4.
Citation1834 Lepidodendron elegans Brongniart; Lindley and Hutton, p. 99, pl. 118.
1837 Lepidodendron elegans (Sternberg) Brongniart; Brongniart, pl. 14.
Citation1848 Lycopodites elegans (Sternberg) Göppert in Bronn, p. 681.
Citation1880 Lepidodendron lycopodioides Sternberg; Zeiller, p. 111 (Zeiller 1878, pl. 171; refigured Zeiller Citation1886, pl. 70).
Citation1947 “Lepidodendron” selaginoides Sternberg; Němejc (non Sternberg), pl. 2, ; pl. 3, .
1959 Sublepidodendron lycopodioides (Sternberg) Remy and Remy, p. 100; fig. 79.
Citation2007 Lepidodendron lycopodioides Sternberg; Opluštil et al., pl. 5, .
2009 Lepidodendron lycopodioides Sternberg; Opluštil et al., .
Citation2021 Lepidodendron lycopodioides Sternberg; Kvaček et al., p. 98, pl. 32.
Lectotype (designated here):
Národní muzeum (Prague), Specimen No. E 4743 (; photographically refigured by Němejc Citation1947, pl. 2, , pl. 3, ; Kvaček et al. Citation2021, pl. 32); Provenance, Svinná, Bohemia (Czech Republic); Whetstone Horizon ash bed (upper Duckmantian). This is the only one of Sternberg’s syntypes of this species that can now be located (Kvaček et al. Citation2021).
Description
Long, slender leafy shoots with narrow leaves that are closely arranged on the stem. Individual leaves are 5‒7 mm long, and 1 mm broad, narrowly lanceolate and gradually acuminate. The leaves open out, then incurve from about their middle.
Remarks
There is some similarity with L. dilatatum (e.g. Álvarez-Vásquez et al. Citation2018) but the leaves of L. lycopodioides tend to be straighter or slightly curving away from the axis, rather than S-shaped with the distal part of the leaf tending to be more parallel to the axis. There is also some similarity with L. selaginoides but the leaves of the latter tend to be more closely adpressed to the stem.
The name Lepidodendron lycopodioides was first used by Sternberg (Citation1820) for lycopsid branches and leafy shoots from the Whetstone Horizon of Svinná. Sternberg (1826) later transferred these specimens to the genus Lycopodiolithes but in doing so he also illegitimately changed the species epithet to elegans. Brongniart (Citation1828) disagreed with this generic distinction but accepted Sternberg’s illegitimate species epithet, and so created the combination Lepidodendron elegans. The latter name continued to be used until Zeiller (Citation1880) recognised the error and re-established the name Lepidodendron lycopodioides.
Lindley and Hutton (Citation1831) later figured a very similar specimen to the L. lycopodioides type as Lepidodendron sternbergii Brongniart Citation1828. As pointed out by Fischer (Citation1904), however, this was illegitimate as L. sternbergii is a later homotypic synonym of Lepidodendron dichotomum Sternberg 1820 (see also Thomas Citation1970). Álvarez-Vásquez et al. (Citation2018) assigned this Lindley and Hutton specimen (also that figured in Citation1834 as L. elegans) to L. dilatatum but their leaves are far straighter than are normally seen in that species.
Lesquereux (Citation1880) followed Lindley and Hutton (Citation1831) in the misuse of the name L. sternbergii for this species, despite the fact that he recognised that its type was conspecific with the type of the earlier published L. lycopodioides; no reason was given for not using the earlier legitimate name. In addition, Lesquereux included the type of Lepidodendron gracile Lindley and Hutton 1830 in this species, but which we believe belongs to Lepidodendron ophiurus (see later), and a specimen figured by Lindley and Hutton (Citation1831-1833b) as L. selaginoides Sternberg, which we have retained in that species (also see later). A study by BAT of Lesquereux’s specimens in the Smithsonian Institution has shown that most of those identified as L. sternbergii are leafy shoots very similar to L. lycopodioides¸ although some have distinctive leaf cushions that enable them to be transferred to Lepidodendron andrewsii Lesquereux 1880 and Lepidodendron cuspidatum Lesquereux 1880 (Thomas et al. Citation2019).
We do not accept Unger’s (Citation1850) inclusion of L. lycopodioides as a synonym of Lepidodendron obovatum Sternberg 1820, the latter being a synonym of the rather different Lepidodendron aculeatum Sternberg 1820 (see Nĕmejc Citation1947; Thomas Citation1970).
Remy and Remy (Citation1959) transferred this species to Sublepidodendron Nathorst in Hirmer Citation1927 as they regarded the stems as being elingulate. However, this was because the upper part of the leaf cushions tends to be obscured by the persistent leaves.
Occurrence
Many of the records are unreliable because of various synonyms. The type came from Whetstone Horizon ash bed at Svinná, Bohemia (Bolsovian); Thomas et al. (2020) recorded it from the Duckmantian of North Wales (UK).
Lepidodendron ophiurus (Brongniart) Brongniart, Citation1828
Figure 9. Lepidodendron ophiurus (Brongniart) Brongniart. (a), Drawing of leafy shoot by Deborah Spillards (from Cleal and Thomas Citation1994). (b), Muséum national d’Histoire naturelle (Paris), Specimen F.1327 (holotype); Newcastle upon Tyne, Tyneside, UK; Pennines Coal Measures. All scale bars = 10 mm.
Citation1822 Sagenaria ophiurus Brongniart, p. 27, pl. 4, .
Citation1825 Lycopodiolithes ophiurus (Brongniart) Sternberg, p. ix.
Citation1825 Lycopodiolithes affinis Sternberg, p. ix, pl. 56, .
Citation1828 Lepidodendron ophiurus (Brongniart) Brongniart, p. 85.
1830 Lepidodendron gracile Lindley and Hutton, p. 29, pl. 9.
1947 Lepidodendron ophiurus (Brongniart) Brongniart; Němejc, pl. 1, ; pl. 2, .
1964 Lepidodendron ophiurus (Brongniart) Brongniart; Crookall, p. 287, text-fig. 93; pl. 61, ; pl. 62, ; pl. 63, .
Citation2015b Lepidodendron ophiurus (Brongniart) Brongniart; Thomas and Seyfullah, .
Holotype
Muséum national d’Histoire naturelle (Paris), Specimen No. 1327 (). Provenance, Newcastle upon Tyne, Northumberland (now Tyneside) UK; Pennines Coal Measures.
Description
Stems with longitudinally elongated, rhomboidal to fusiform leaf cushions with acute upper and lower angles and rounded lateral sides. The leaf laminae are attached about one-third down the leaf cushions. Keels are above and below the attachment area of the leaf laminae and lateral lines run from the attachment area to the cushion edges. The leaves are linear, single veined, gradually tapering to an acute point, attached to cushions at nearly right angles, but then directed upwards with only slight divergence from the stem axis (based on Thomas and Seyfullah Citation2015b).
Remarks
The taxonomy of this species has been resolved by Thomas and Seyfullah (Citation2015b). The latter authors included L. dilatatum Lindley and Hutton as a synonym of L. ophiurus, but we now reject this as the former has leaves more S-shaped leaves that are acutely attached to the stem.
Álvarez-Vásquez et al. (Citation2018) assigned the type of L. gracile Lindley and Hutton 1831 to L. dilatatum. However, the leaves are clearly attached at a very obtuse angle to the leaf cushion and are far more similar to L. ophiurus.
Bureau (Citation1913, pl. 30, ; pl. 36, ; pl. 37, ) figured several leafy shoots as L. ophiurus from the upper Mississippian of northern France. As pointed out by Strullu-Derrien et al. (Citation2021), however, the leaves are rather longer and thicker, and are not as consistently curved at the end. We know of no examples of L. ophiurus from earlier than the Pennsylvanian.
Occurrence
In the UK (e.g. Thomas et al. 2020): Coal Measures of Durham; from above the Barnsley Coal, East Gawber Colliery, near Barnsley, West Yorkshire (Duckmantian); from the Percy colliery, Newcastle-on-Tyne above the Sourmilk Coal, New Caledonian Railway, Airdrie, West Lothian (Bolsovian); Crookall (Citation1964; Citation1966) stated it to be a very common and widespread, but the records are unreliable because of his broad interpretation of the species; Thomas (Citation2007) recorded the species from the Asturian of Southern Britain, Sydney coalfield in Canada, Bohemia in the Czech Republic and the Dobrudzha coalfield in Bulgaria.
Lepidodendron pictoense Dawson Citation1863
Citation1863 Lepidodendron pictoense Dawson, p. 449.
Citation1866 Lepidodendron pictoense Dawson; Dawson, p. 160, pl. 9, fig. 37.
Citation1868 Lepidodendron pictoense Dawson; Dawson, pp. 487, 453, fig. 169A.
Citation1880 Lepidodendron lanceolatum Lesquereux, p. 369 (1879, pl. 63, ).
Citation1962 Lepidodendron pictoense Dawson; Bell, p. 52, pl. 49, , pl. 50, .
1966 Lepidodendron pictoense Dawson; Bell, pl. 29, .
Lectotype (designated here):
Redpath Museum, McGill University, Specimen 3478 (). Provenance, Sydney Coalfield, Nova Scotia (Canada); Morien Group (Asturian). Originally figured by Dawson (Citation1866, pl. 9, fig. 37a) and Dawson, Citation1868, fig. 169A).
Description
Leaf cushions about four times longer than broad, with acute upper and lower angles, and broadly rounded lateral sides with the leaf scars in the upper half of the cushion. Ligule pit apertures about 1 mm above the leaf scars., The leaves are 10‒25 mm long and 1.5‒3 mm broad, bending out to about 45° and then straight or more generally curved upwards. (Based on Bell Citation1962).
Remarks
Dawson (Citation1863) described Lepidodendron pictoense as a new species without illustrating types, but then later (Dawson Citation1866, Citation1868) repeated the description with accompanying drawings of a leafy shoot. Bell (Citation1962) regarded Dawson’s descriptions inadequate for a satisfactory diagnosis and gave an emended description. In our view, the relatively straight leaves attached to the stem at a narrow angle in the somewhat wider stems (e.g. ) are quite different from the other species recognised here. As pointed out by Bell (Citation1962) the different leaf sizes seen in the syntypes were said to relate to the size of the branches, which fits in with modern ideas of the growth pattern of the plants.
We agree with Bell (Citation1962) that the types of Lepidodendron lanceolatum Lesquereux, 1879 are identical to those of L. pictoense.
Occurrence
Abundant in the Morien Group (Bolsovian ‒ Cantabrian) of the Sydney and Pictou coalfields of the Canadian Maritimes (Dawson Citation1866); Bell’s (Citation1966) figured specimens were from the Bolsovian Clifton Formation of the Pictou Coalfield. Lesquereux’s (Citation1879) type of L. lanceolatum originated from the Clinton Coal of Missouri (USA).
Lepidodendrom rigens Lesquereux Citation1870
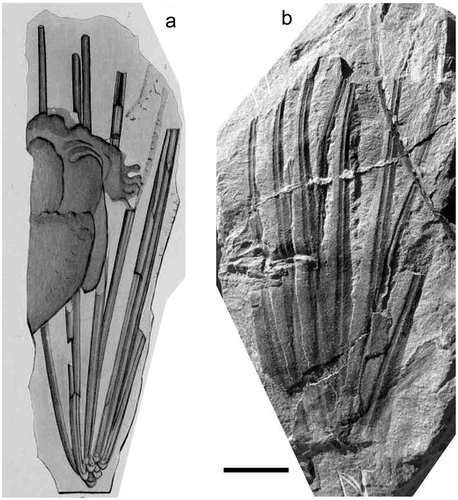
Figure 10. Lepidodendrom rigens Lesquereux (lectotype), Smithsonian Museum of Natural History, Specimen No. 15516, Mazon Creek, Illinois (USA). (a), Original illustration given with protologue in Lesquereux (Citation1870). (b), Photograph of lectotype. Scale bar = 10 mm.
Citation1870 Lepidodendron rigens Lesquereux, p. 429, pl. 27, .
Citation1880 Lepidodendrom rigens Lesquereux, p. 372.
Citation1884 Lepidodendron rigidum Lesquereux, p. 839.
Citation1925 Lepidodendron rigens Lesquereux; Noé, pl. 9.
Citation1979 Lepidodendron rigens Lesquereux; Janssen, fig. 22.
Lectotype (designated here):
Smithsonian Museum of Natural History, Washington, Specimen No. 15516 (); Provenance, Mazon Creek, Illinois (USA); Middle Pennsylvanian (Cantabrian). Other syntypes in the Lesquereux collections are Specimen Nos. 15515 and 15517‒15557.
Description
Branches with long rigid sub-cylindrical laminae, more than 200 mm long and 3 mm in diameter, gradually acuminate attached to rhomboidal, laterally enlarged leaf cushions.
Remarks
Lesquereux (Citation1870) remarked that the leaf cushions were not visible but later (1880) described them as upraised, rhomboidal and laterally enlarged. The leaf scars covered the upper half of the cushions. This might infer that the leaf cushions were of the Lepidophloios type and, if Lesquereux had mistakenly inverted the specimen, this might be true. However, he does describe leaves, so he must have known which was up for the leaf cushions. For further discussion on the problem of leaf cushion inversion see Thomas (Citation1977).
Lesquereux (Citation1884) believed the leaves of L. rigidum to resemble those of his L. rigens, but were shorter and only slightly concave towards the base. This difference in shape could be a preservation effect and the leaf length difference the result of their relative positions on the parent plants. For those reasons we regard the two species as synonyms.
The only European record that has been suggested as belonging to this species was the specimen figured by von Röhl (Citation1868, pl. 11, ) as Lepidodendron dichotomum Sternberg (Jongmans Citation1929). However, the isodiametric leaf cushions make it more similar to L. latifolium.
Occurrence
Mazon Creek, near Morris, Grundy County, Illinois; Tennessee, USA (Cantabrian) (Lesquereux, Citation1870; Noé Citation1925; Janssen Citation1979); Pottsville, Pennsylvania from the Dade Mine, Georgia, USA (stratigraphical age uncertain) (Lesquereux Citation1884).
Lepidodendron selaginoides Sternberg Citation1821
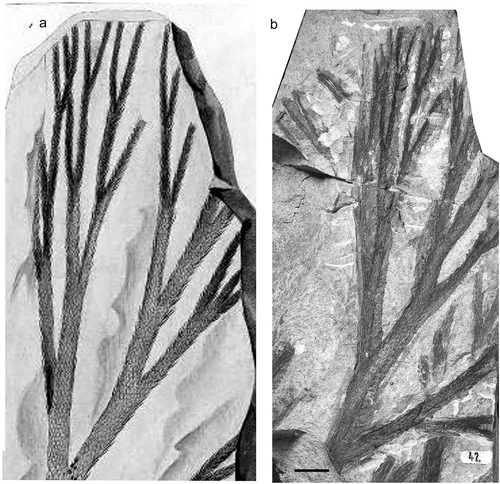
Figure 11. Lepidodendron selaginoides Sternberg, Národní muzeum (Prague), Specimen E 4744 (lectotype); Svinná, Czech Republic; Whetstone Horizon (Duckmantian). (a) Original illustration from Sternberg (Citation1821). (b), photo by Lenka Váchová; scale bar = 10 mm.
1821 Lepidodendron selaginoides Sternberg, p. 31, pl. 16, ; pl. 17, .
Citation1825 Lycopodiolithes selaginoides (Sternberg) Sternberg, p. viii.
Citation1831 Lepidodendron selaginoides Sternberg; Lindley and Hutton, pl. 12.
1855 Lycopodites selaginoides (Sternberg) Geinitiz, p. 33, pl. 1, .
Citation1868 Lycopodites selaginoides (Sternberg) Geinitiz; von Röhl, p. 144, pl. 6. ; pl. 7. .
Citation1875 Lycopodium carbonaceum Feistmantel, p. 183, pl. 30, .
Citation1947 “Lepidodendron” selaginoides Sternberg; Nĕmejc, pl. 3, .
1997 Lepidodendron selaginoides Sternberg; Kvaček and Straková, p. 138, pl. 50, .
Lectotype (designated here):
Národní muzeum (Prague), Specimen No E 4744. Provenance, Svinná, Bohemia (Czech Republic); Whetstone Horizon ash bed (upper Duckmantian) (refigured photographically Nĕmejc Citation1947, pl. 3, ; Kvaček et al. Citation2021, pl. 50, ).
Description
Narrow stems with leaves that are linear, straight, short, and almost adpressed to the stem. Stems dichotomise at very narrow angles; largest known stem 30 mm wide (Nĕmejc Citation1947).
Remarks
Kidston (Citation1889) and Crookall (Citation1964) described L. selaginoides shoots bearing the cone now known as Flemingites olryi (Zeiller) Brack-Hanes and Thomas Citation1983, but using the name Bothrodendron minutifolim Boulay Citation1876. As a consequence, Kidston (Citation1911) regarded B. minutifolium as conspecific with L. selaginoides, quoting the figures of Lindley and Hutton (Citation1831) and von Röhl (Citation1868); this view was also followed by Bureau (1914) and Jongmans (Citation1929). However, Chaloner (Citation1953) and Thomas (Citation1967a) showed that leafy shoots in organic connection with large non-leafy stems identifiable as B. minutifolium (Zeiller Citation1886, pl. 74, ; Lindsey Citation1915, pl. 2, ) have broader, more ovate and squarrose leaves than those attached to the F. olryii strobili; epidermal characters also serve to distinguish the two types of leafy shoot. We now believe the latter to be shoots referrable to L. selaginoides, making this a true species.
There has also been a tradition of using this name for anatomically preserved lycopsid shoots such as found in coal balls, following Carruthers (Citation1869) and Williamson (Citation1872). As pointed out by Jongmans (Citation1929), however, there is no actual evidence that these anatomically preserved shoots correspond in morphology to the adpression types of L. selaginoides and so the use of that name for the anatomically preserved shoots is unwarranted.
Occurrence
Langsettian – Bolsovian of Bohemia, Czech Republic (Sternberg Citation1821, Citation1825); the roof of the Low Main coal seam (Duckmantian), Felling Colliery, Gateshead, Tyne and Wear, UK (Lindley and Hutton 1831).
Lepidodendron wingfieldense Hemingway ex Thomas and Seyfullah Citation2015b
Figure 12. Lepidodendron wingfieldense Thomas and Seyfullah (holotype), British Geological Survey, Keyworth, Specimen 76,267, Wingfield Colliery, Derbyshire (UK); above the Kilburn Coal (Langsettian).

Citation2015b Lepidodendron wingfieldense Hemingway MS; Thomas and Seyfullah, p. 31, text .
Holotype
British Geological Survey, Keyworth (UK), Specimen No. 76267. Provenance, Wingfield Colliery, Derbyshire (UK); above the Kilburn Coal (Langsettian).
Description
Stems variable in size, up to at least 62 mm in diameter with acute branching at 10‒20°. The leaf cushions are up to 5.9 mm long and 4.8 mm broad with acute pointed upper and lower angles and rounded sides and are distinctly grooved along the median axis. The leaf laminae depart from about a third of the distance from the base apex, c. 4 mm long with acute apices.
Remarks
Crookall (Citation1964) included within L. ophiurus some specimens that Hemingway had collected and given the manuscript name of L. wingfieldense. However, Thomas and Seyfullah (Citation2015b) showed that these had leaves that were attached at a much more acute angle than in L. ophiurus and used Hemingway’s manuscript name of L. wingfieldense, giving the species the description quoted above.
Striations on the leaf cushions of L. wingfieldense distinguish it from most other species of Lepidodendron. The most similar is L. worthenii Lesquereux, but the cushions of the latter are always elongated with much more prominent grooves running much further across the leaf cushions. The leaf laminae on L. worthenii are also much longer and more distinct than those of L. wingfieldense. The types of L. wingfieldense is much older (Langsettian) than L. worthenii, which is most commonly found in the Asturian. The slenderer shoots of L. wingfieldense can also resemble L. selaginoides but the latter lacks the striate markings on the proximal parts of the leaves; furthermore, the stems of L. selaginoides shoots never reach the thickness regularly seen in L. wingfieldense.
Occurrence
The only known occurrence of the species is from the Langsettian type locality in Derbyshire, UK
Lepidodendron worthenii Lesquereux Citation1866
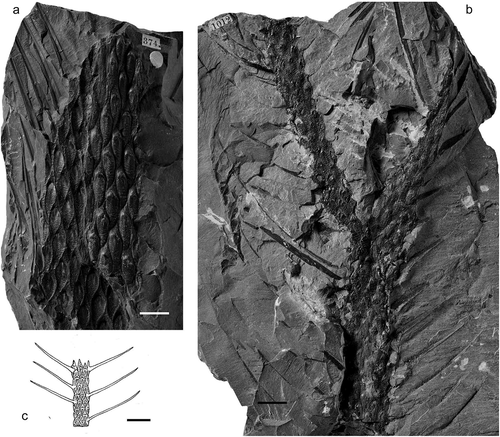
Figure13. Lepidodendron worthenii Lesquereux. (a), British Geological Survey, Kidston Collection Specimen 379, unknown locality in UK; Coal Measures (Westphalian). (b), British Geological Survey, Kidston Collection Specimen 1012, Gawber Colliery, Barnsley, Yorkshire (UK); Middle Coal Measures (Duckmantian). (c), Drawing of leafy shoot by Deborah Spillards (from Cleal and Thomas Citation1994). All scale bars = 10 mm.
Citation1866 Lepidodendron wortheni Lesquereux, p. 452, pl. 44, .
Citation1880 Lepidodendron wortheni Lesquereux; Lesquereux, p. 388 (Lesquereux Citation1879, pl. 64, ).
Citation1880 Lepidodendron brittsii Lesquereux, p. 368 (Lesquereux Citation1879, pl. 63, ).
Citation1938 Lepidodendron wortheni Lesquereux; Bell, p. 94, pl. 96, .
Citation1940 Lepidodendron wortheni Lesquereux; Janssen, p. 13, pl. 1, .
Citation1979 Lepidodendron wortheni Lesquereux; Jannsen, p. 43, fig. 19.
1964 Lepidodendron wortheni Lesquereux; Crookall, p. 275, pl. 51, .
Holotype
Illinois State Museum, Springfield IL, Specimen No. 1730. Provenance, Murphysboro IL (USA); from above coal no. 1B (photographically refigured by Janssen Citation1940, pl. 1, ; Citation1979, fig. 19)
Description
Leaf cushions with deep horizontal grooves, both above and below the leaf scars. The characteristic cushions can be seen on leafy shoots where the leaf laminae are straight and attached at c. 45‒50° from the vertical. Individual laminae are about 20‒60 mm long, presumably varying in length reflecting their relative positions on the branches and narrowing upwards to a sharp point.
Remarks
The taxonomy of this species has been discussed by Thomas et al. (Citation2019) including its relationship with Lepidodendron brittsii Lesquereux, 1880. See also the comments above on L. wingfieldense.
Occurrence
Most of the records of this species come from the Asturian and has been recorded from the Pennsylvanian of Illinois, Pennsylvania, Indiana; Oklahoma, New Mexico in the USA; Nova Scotia in Canada (for further details of the American records see Álvarez-Vásquez and Wagner Citation2014); South Wales, Forest of Dean, Bristol & Somerset the Forest of Wyre and South Staffordshire in the UK; the Donetz Basin in the Ukraine; Dobrudzha Coalfield, Bulgaria and in Spain and Portugal (Thomas Citation2007). Josten (Citation1991, fig. 76, pl. 43 ) described and figure specimens from north-west Germany that we can accept. The figured specimens are from the Duckmantian although Josten also recorded it from the Langsettian and the Bolsovian of the Ruhr coalfield, the Langsettian and Duckmantian of the Aachen coalfield. Van Ameron (1975) recorded the species from the Netherlands. Crookall (Citation1964) recorded and figured (pl. 61) a specimen of this species from the Bench Coal of Warwickshire, U.K. Close examination of the specimen shows its leaf cushions to be completely covered with horizontal striations, but they have distinct triangular leaf scars with three foliar prints a feature never seen on Asturian specimens of L. worthenii.
Genus: Lepidophloios (“Lepidofloyos”) Sternberg Citation1825 nom. cons.
Remarks
The nomenclatural status and conservation of the name Lepidophloios has been discussed by Wang (Citation2007a, Citation2007b) and Doweld (Citation2013). The genus is characterised by having downward bulging leaf cushions where the leaf scar occurs at the base of the bulge. Leafy stems can only be attributed to one species, L. acerosus.
Lepidophloios acerosus (Lindley and Hutton) Kidston Citation1890
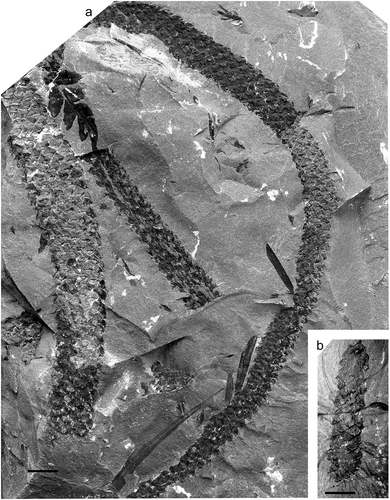
Figure 14. Lepidophloios acerosus (Lindley and Hutton) Kidston, National Museum Wales, Specimen NMW 2013.43 G.136; Brymbo Opencast near Wrexham, Denbigh Coalfield, north Wales, UK (photo by P. Appleton); Middle Coal Measures (Duckmantian).
Citation1831 Lepidodendron acerosum Lindley and Hutton, p. 29, pl. 7, ; pl. 8.
Citation1837 Lepidostrobus pinaster Lindley and Hutton, p. 129, pl. 198.
Citation1838 Lycopodites acerosus (Lindley and Hutton) Presl in Sternberg, p. 176.
Citation1890 Lepidophloios acerosus (Lindley and Hutton) Kidston, p. 49.
Citation1893a Lepidophloios acerosus (Lindley and Hutton) Kidston; Kidston, p. 558, pl. 1, ; pl. 2, .
Citation1904 Lepidophloios acerosus (Lindley and Hutton) Kidston; Kidston, text-fig7c.
Citation1914 Lepidophloios acerosus (Lindley and Hutton) Kidston; Arber, p. 414, pl. 28, fig. 20.
Citation1917 Lepidophloios acerosus (Lindley and Hutton) Kidston; Kidston, pl. 2, .
Citation1929 Lepidodendron acerosus Lindley and Hutton; Crookall, p. 26, pl. 3, fig. 50; pl. 22, fig. k.
1964 Lepidophloios acerosus (Lindleyand Hutton) Kidston; Crookall, p. 313, pl. 75 ; pl. 76, .
Citation1977 Lepidophloios acerosus (Lindley and Hutton) Kidston; Thomas, p. 280, pl. 35, ; text-.
Syntypes
Lindley and Hutton (Citation1831, pl. 7, ; pl. 8). Provenance, Bensham Colliery, roof of the Bensham Seam (pl. 7, ) and Felling Colliery, Low Main Seam (pl. 8); both of Duckmantian age, and located at Gateshead, Tyne and Wear (UK). According to Kidston (Citation1891, Citation1893a) all syntypes are lost.
Description
Stems with downturned and overlapping leaf cushions whose exposed parts are longer than broad. The cushions have clear keels that are usually slightly raised. The leaf scars are at the base of the downturned leaf cushions and ligule pit apertures occur just above them. Leaves are 40‒50 mm long and ascend from the cushions at narrow angles. Epidermal structure different on the exposed upper surface of the leaf cushion and its lower hidden surface.
Remarks
The interpretation of this species is problematic as all of the syntypes are reported lost and the figures provided by Lindley and Hutton (Citation1831) are somewhat diagrammatic. We have provisionally followed the interpretation of the species by Kidston (Citation1893a) and Thomas (Citation1977) as a more complete taxonomic analysis will be required of stems in all growth stages will be needed before a neotype can be selected.
Lindley and Hutton (Citation1837) figured what they interpreted as a strobilus as Lepidostrobus pinaster (). However, this is clearly a stem with downturned leaf cushions and attached leaves, and belongs to L. acerosus.
The leaf cushions on the smallest shoots of this species are not downturned and resemble those of Lepidodendron. Thomas (Citation1977) has shown that some stems (e.g. Nos 764 and 4947 in the Kidston collection, British Geological Survey) show a progression from smaller un-downturned to larger downturned cushions and suggested that this was subsequent growth possibly to increase photosynthesis. Leaves were shed before the expansion of the leaf cushions.
Occurrence
This has been recorded from the Langsettian to the Bolsovian of England and Wales (Crookall Citation1964), the Duckmantian of North Wales and the Asturian of Southern Britain by Thomas (Citation2007); Cucuiova Formation of the Sirinia Basin, Danubian Units, in Almăj Mountains, South Carpathians (Bolsovian ‒ Cantabrian) in Romania (Popa Citation2016); Bolsovian of the Czech Republic (Opluštil et al. Citation2009b).
Genus: Ulodendron Lindley and Hutton Citation1831
Type: Ulodendron majus Lindley and Hutton 1831
Remarks
Jongmans (Citation1913) attributed the publication of this genus name to Rhode (Citation1823), mainly following Lindley and Hutton (Citation1831) who regarded the specimen figured by Rhode as being conspecific with their Ulodendron majus. However, Rhode never actually used the name, whose first valid use was by Lindley and Hutton (1831).
Ulodendron is characterised by having rhomboidal leaf cushions separated by narrow grooves and narrow areas of flat stem above each cushion. The leaves were retained so no true leaf scars occur. Ligule pits are present at the base of the adaxial leaf lamina (Thomas Citation1967b, ). The large round so-called ulodendroid branch-abscission scars illustrated by Lindley and Hutton (Citation1831) are not diagnostic features for Ulodendron, having also been found on stems with leaf scars referrable to other fossil-genera including Lepidodendron and Synchysidendron (e.g. Bureau Citation1913; DiMichele et al Citation2013).
Renier (Citation1926) used Ulodendron for all lycopod leafy shoots, and this was followed by Moore and Cox (Citation1943), and Moore (Citation1945). Stockmans and Willière (Citation1953) described cones attached to lycopod leafy shoots as Ulostrobus, but there was no evidence that the shoots were in fact Ulodendron and these strobili are indistinguishable from Lepidostrobus and Flemingites.
Pearson (Citation1986) interpreted Ulodendron as a synonym of the genus of anatomically preserved stems Anabathra, and proposed new combinations and species previously assigned to Ulodendron. However, this is an example of the confusion that can occur when attempts are made to integrate the fossil-taxa in different states of preservation (see comments by Thomas and Cleal Citation2020). Anabathra was based on a Tournaisian fossil from Scotland (Witham Citation1833). Pearson suggested that they had a similar anatomy to Visean stems now named Paralycopodites brevifolium (Williamson) DiMichele Citation1980 and that the latter species is equivalent to the Pennsylvanian-age Ulodendron adpressions based on the presence of large branch scars on the stems (DiMichele Citation1980). However, these scars are now known not to be a reliable indicator of that genus (see above). There is, moreover, now doubt that Anabathra and Paralycopdites are generically the same (DiMichele and Bateman Citation1996). Since there is no clear evidence that the types of either Anabathra or Paralycopodioides have the same diagnostic morphological features as the rather younger Ulodendron adpressions, and there is similarly little evidence that the type of Ulodendron had the Anabathra/Paralycopodites anatomy, combining these fossil-genera merely introduces confusion and lack of clarity into the taxonomy, and should be rejected.
Ulodendron majus Lindley and Hutton 1831
Figure 15. Ulodendron majus Lindley and Hutton. (a), British Geological Survey, Kidston Collection Specimen 19, Devonside, Tillicoultry, Clackmannanshire (UK); Lower Coal Measures (Duckmantian); scale bar = 10 mm. (b), Drawing of leaf cushions with associated leaves (from Thomas Citation1967b).
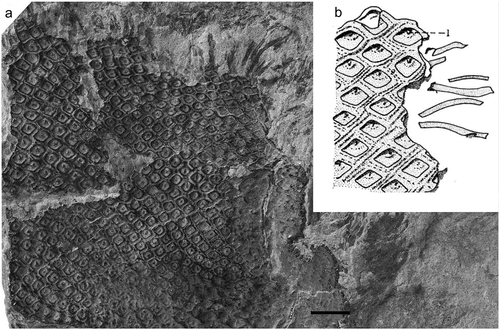
Citation1825 Lepidodendron discophorum König (nomen nudum), pl. 16, fig. 194.
Citation1831 Ulodendron majus Lindley and Hutton, p. 22, pl. 5.
Citation1831 Ulodendron minus Lindley and Hutton, p. 25, pl. 6.
Citation1885a Sigillaria discophora (König) Kidston (nom. illegit.), p. 251, pl. 4, ; pl. 5, ; pl. 7, .
Citation1886 Ulodendron majus Lindley and Hutton; Zeiller, pl. 73.
Citation1966 Ulodendron majus Lindley and Hutton; Crookall, p. 486, pl. 99, ; pl. 100, ,
1967b Ulodendron majus Lindley and Hutton; Thomas, p. 778, .
Citation1986 Anabathra thomasiana Pearson, p. 280.
Holotype
Lindley and Hutton (Citation1831, pl. 5). Provenance, Jarrow Colliery, near Newcastle-upon-Tyne (UK); Bensham Seam (Duckmantian). The holotype has been reported lost but there are 13 other specimens in the Hutton Collection from the same locality from which a neotype might be selected (Lebour Citation1878). However, such a selection would need the specimens to be investigated.
Description
Leaf cushions rhomboidal and slightly broader then long, and separated by narrow grooves. The laminae are c. 30 mm long and 1.5 mm broad at their attachment points.
Remarks
The holotype is lost and the illustration accompanying protologue in Lindley and Hutton (1831) is somewhat unclear. However, the latter seems to suggest that the stems had rhomboidal leaf cushions, slightly broader than long, and lacking leaf scars, and this was the basis of the interpretation of this species by Thomas (Citation1967b) and which we have followed here. Thomas (Citation1967b, ) showed a stem with associated elongated leaves, and a similar specimen has been found in the Kidston Collection (). A specimen with attached leaves was figured by Zeiller (Citation1886); Álvarez-Vásquez et al. (Citation2018) identified the latter as Lepidodendron dilatata but the leaves are much longer and straighter than in that species, and the squat, rhomboidal leaf cushions clearly correspond to those of U. majus.
Ulodendron minus was mainly differentiated by having smaller leaves and leaf scars (Lindley and Hutton Citation1831; Zeiller Citation1888). However, this almost certainly represents parts of the same plant species (Thomas Citation1967b).
Kidston (Citation1885a, Citation1885b) named the species Sigillaria discophora (König) Kidston (≡ Lepidodendron discophora König 1825). However, the basionym was only given as a label on a figure, with no accompanying description or text, and so was not validly published.
Pearson (Citation1986) proposed that the species as interpreted by Thomas (Citation1967b) should be re-named Anabathra thomasiana as the U. majus type is lost and it is not certain that the latter is conspecific with Thomas’s material. However, we regard this as premature as there are paratypes in the Hutton Collection that could be used to establish the true nature of U. majus. We have therefore continued to use the epithet majus as proposed by Lindley and Hutton.
Occurrence
U. majus has been recorded from above the Bensham Seam, Jarrow colliery, Tyne and Wear, UK; and above the High Main coal South Shields colliery Tyne and Wier, UK; also from Kilmarnock, Ayrshire, Scotland, UK (Thomas Citation1967b); the Duckmantian of North Wales (Thomas et al. Citation2019).
Ulodendron landsburgii (Kidston) Thomas Citation1968.
Figure 16. Ulodendron landsburgii (Kidston) Thomas. British Geological Survey, Kidston Collection Specimen 4546 (lectotype), Bonnington pit, Kilmarnock, Ayrshire (UK); above the Whistler seam (Bolsovian). Scale bar = 10 mm.

Citation1893b Lepidodendron landsburgii Kidston, p. 338, pl. 3, .
1964 Lepidodendron ophiurus Brongniart; Crookall (pars, non Brongniart), p. 287, pl. 63, .
Citation1968 Ulodendron landsburgii (Kidston) Thomas, p. 425, .
Citation1986 Anabathra landsburgii (Kidston) Pearson, p. 280.
Holotype
British Geological Survey, Keyworth (UK), Kidston Collection, Specimen No. 1546. Provenance, Bonnington pit, Kilmarnock, Ayrshire (UK); from above the Whistler seam (Bolsovian).
Description
Leaf cushions longer than broad and separated by narrow grooves. Laminae are c. 25‒30 mm long and 2 mm broad at their attachment points.
Remarks
Crookall (Citation1964) assigned the type of this species to L. ophiurus but it is clearly a Ulodendron based on both morphological and epidermal characters (Thomas Citation1967b). Thomas (Citation1968) showed that it differed from U. majus by having more elongate cushions and the larger, sunken stomata.
Álvarez-Vásquez and Wagner (Citation2014) regarded U. landsburgii as a synonym of Bergeria dilatata, but in our view they have different shaped leaves.
Occurrence
This species is known only from the type locality in Ayrshire.
Bothrodendron Lindley and Hutton1833a
Type: Bothrodendron punctatum Lindley and Hutton Citation1833a.
Remarks
Being the name of an initially monotypic genus, it was validated by the protologue of the type species. Lindley and Hutton (Citation1834, p. 97) later gave a generic diagnosis (“Stem not furrowed, covered with dots. Scars of cones, obliquely oval”) but this definition was ambiguous. Following Presl (in Sternberg Citation1838) many authors regarded it simply as a decorticated form of Ulodendron, but Zeiller (Citation1885) and Kidston (Citation1904) showed that this was in error and that the stems were not decorticated, but were simply lacking leaf cushions. Zeiller (Citation1885) also pointed out that a stem figured by Boulay (Citation1876, pl. 3, ) under the genus Rhytidodendron also belonged to Bothrodendron as he was then defining it.
We are here using the fossil-genus with the emended diagnosis given by Thomas (Citation1967a): stems with small leaf scars with three foliar scars that are level with the wrinkled stem surface; ligule pit apertures are immediately above or slightly separated from the leaf scars; smaller leafy branches covered with small acutely pointed leaves.
Bothrodendron is distinguished from the similar fossil-genera Cyclostigma Haughton Citation1859 and Pinakodendron Weiss Citation1893 which show no ligule pits, and from Lepidobothrodendron Daber Citation1959 which has two external parichnos.
Bothrodendron minutifolium (Boulay) Zeiller Citation1880
Figure 17. Bothrodendron minutifolium (Boulay) Zeiller. (a), British Geological Survey, Kidston Collection Specimen 1546 (lectotype), Bonnington pit, Kilmarnock, Ayrshire (UK); above the Whistler seam (Bolsovian). Scale bar = 10 mm. (b), Drawing of leafy shoot by Deborah Spillards (from Cleal and Thomas Citation1994; based on Thomas Citation1967a)

Citation1876 Rhytidodendron minutifolium Boulay, p. 39, pl. 3, , 1bis.
Citation1880 Bothrodendron minutifolium (Boulay) Zeiller, p. 117.
Citation1915 Bothrodendron minutifolium (Boulay) Zeiller; Lindsey, pl. 11.
Citation1967a Bothrodendron minutifolium (Boulay) Zeiller; Thomas, .
Lectotype (designated here):
Boulay (Citation1876, pl. 3, bis). The specimen was probably in the collections of the Muséum national d’Histoire naturelle (Paris), but has not been located. Provenance, Bonne-Part pit, Fresnes, north of Valencienne (France).
Description
Large shoots with oval, laterally elongated leaf scars level with the stem surface and ligule pit apertures just above them. The stem surface is divided into small areas by fine, irregular and undulating grooves with each area containing groups of small puncti (probably stomata ‒ Thomas Citation1967a) in horizontal rows. Smaller shoots have leaf scars on raised leaf cushions whose surface features are like those on the largest shoot, while the smallest, leafy twigs have ovate-lanceolate, leaves that are about 5 mm long with acute pointed apices. The midrib is about 150 µm broad narrowing rapidly towards the leaf apex.
Remarks
Some authors have reported leafy shoots attached to the identifiable stems of B. minutifolium (e.g. Zeiller Citation1880; Weiss Citation1893; Kidston Citation1902; Lindsey Citation1915); the Kidston specimen is refigured here as . However, it is difficult to identify the isolated leafy shoots of B. minutifolium unless they are found attached to diagnostic, wider stems.
Occurrence
B. minutifolium has been recorded by Crookall (Citation1964) as a common and widespread species from the Langsettian to the Duckmantian in many coalfields in England, Scotland and Wales; Thomas (Citation2007) recorded it from the Asturian of Iberia and Bulgaria.
Bothrodendron punctatum Lindley and Hutton Citation1833a
Figure 18. Bothrodendron punctatum Lindley and Hutton. Nova Scotia Museum, Halifax NS, Specimen 990–281), Pioneer Coal Mine at Sydney Airport, Nova Scotia (Canada); Gardiner Seam, Morien Group (middle Asturian) (from Thomas et al. Citation2010, fig. 2b; photo by E.L. Zodrow). Scale bar = 10 mm.

Citation1833a Bothrodendron punctatum Lindley and Hutton, pls 80, 81.
1967a Bothrodendron punctatum Lindley and Hutton; Thomas, p. 53, .
2010 Bothrodendron punctatum Lindley and Hutton; Thomas et al., p. 2, .
Lectotype
British Geological Survey, Keyworth (UK), Specimen No. 5257. Provenance, Jarrow Colliery, near Newcastle-upon-Tyne (UK); from the roof of the High Main Seam (Duckmantian) (Citation1964). The whereabouts of the other syntype figured by Lindley and Hutton (Citation1833a, pl. 81), from the Percy Main Colliery, Newcastle-upon-Tyne, is unknown.
Description
Stems with oval leaf scars up to 2 mm long with ligule pits contiguous with the top of the leaf scar. The stem surface is covered with fine, sinuous, vertical striations, and the epidermis has uniformly elongated epidermal cells and evenly scattered stomata. The side shoot departs upwards at an angle of about 20° with a decurrent upper surface. Side shoots are about 10 mm across a little way from its broader point of attachment and for the first 20 mm bear no leaves. The surface of this basal portion of the branch is like that of the main stem. The leaves are in tight spirals decurrent and then spreading out at angles of 30–40° before curving upwards again. Individual leaves are lanceolate, about 0.6 mm long and about 0.2 mm across at their broadest point and their apices are shortly acuminate.
Remarks
The above description of a leafy side shoot is based on a specimen from above the middle Asturian Gardiner Seam of the Sydney Coalfield, Nova Scotia, described by (Thomas et al. Citation2010). The leaves of B. punctatum are very similar to those of B. minutifolium but are less densely packed together on the shoots.
Occurrence
The type specimen came from the Duckmantian of the UK; Crookall records the species from the UK, from the Langsettian (Yorkshire, Derbyshire, Lancashire, and Lanarkshire), and Bolsovian to Asturian (South Wales, Yorkshire, Derbyshire, Nottinghamshire, Northumber-land and Durham, North Ayrshire, Clackmannanshire and Fife. Thomas (Citation2007) recorded it from the Asturian of Nova Scotia, Canada and Bulgaria.
1. Large stems with small leaf scars level with the stem surface that is wrinkled. The smaller branches are leafy and covered with small acutely pointed leaves 2
Stems with leaf cushions 3
2. Main stems horizontally wrinkled. Leaves on smaller branches ovate-lanceolate about 5 mm long with acute pointed apicesBothrodendron minutifolium
Main stems vertical striated. Leaves on smaller branches lanceolate, about 0.6 mm long and shortly acuminateBothrodendron punctatum
3. Leaf cushions rhomboidal separated by narrow grooves and areas of flat stem 1 mm broad above each leaf cushion. Leaf laminar broadly attached and never shed 4
Leaf cushions elongated or not visible being covered by narrow laminae 5
4. Leaf cushions slightly broader than long, laminae c. 30 mm long and 1.5 mm broad at their attachment pointsUlodendron majus
Leaf cushions slightly longer than broad, laminae 25‒30 mm long and 2 mm broad at their attachment pointsUlodendron landsburgii.
5. Leaves attached at right angles to the leaf cushions 6
Leaves attached at acute angles to the leaf cushions 7
6. Leaves turned upwards to be more or less parallel to shoot axis Lepidodendron ophiurus.
Leaves curved upwards and outwards Lepidodendron dawsonii.
7. Leaves S-shaped at their base and curved in the upper parts often reaching 50‒70 mm in length. If laminae are missing a curved lines visible near the apices are pseudoscarsLepidodendron dilatatum.
Leaves straight or curved and departing from leaf cushions 8
8. Leaves attached at c. 45° to leaf cushions Lepidodendron pictoense.
Leaves attached at less than 45° to leaf cushions9
9. Leaves over 40 mm long 10
Leaves 35 mm long or less 13
10. Leaves cylindrical, >200 mm long, 3 mm in diameterLepidodendron rigens.
Leaves flat not cylindrical 11
11. Leaves 40‒50 mm long, attached to diamond-shaped cushions at narrow angles Lepidophloios acerosus
Leaves attached to shoots with no obvious leaf cushions 12
12. Leaves less than 5 mm broadLepidodendron longifolium.
Leaves 7 mm broadLepidodendron latifolium.
13. Leaves 10‒25 mm long, 1.5‒3 mm wide, bending outwards to about 45° and then curving upwardsLepidodendron pictoense
Leaves less than 10 mm long 14
14. Narrow stems with closely arranged leaves and no obvious leaf cushions 15
Broad stems with leaf cushions with a striate surface, and leaves c. 4 mm long with acute apicesLepidodendon wingfieldense
15. Leaves spreading, 5‒9 mm long and 1 mm wide, lanceolate and gradually narrowing Lepidodendron lycopodioides
Leaves straight, up to 5 mm long, less than 1 mm wide and closely adpressed to the stemLepidodendron selaginoides
Key for distinguishing species of leafy shoots
Discussion
We have shown that leafy shoots of arborescent lycopods can be identified using leaf size and shape. Then, considering the growth patterns of the plant, it is possible to interpret very similar species as synonyms. For example, we have synonymised Bergeria acuta into Lepidodendron dilatatum, and Ulodendron minus into U. majus, regarding both pairs of species as expressions of different sized stems. It has also been possible to untangle the taxonomic confusion over leafy shoots sometimes described as Bothrodendron minutifolium with those having terminal cones of Flemingites olryi; the latter have been shown to be identical with, and better described as, Lepidodendron selaginoides.
We have not attempted to integrate the adpression taxa of leafy lycopod shoots with taxa of the anatomically preserved leaves described by Graham (Citation1935); the latter species tended to be differentiated on characters, such as the relative distribution of tissue-types and of stomatal furrows seen in transverse sections, which are virtually impossible to recognise in adpressions. Similarly, the morphological characters used to differentiate the leaf adpression species would be very difficult to recognise in anatomically preserved fossils.
As with the stems, trying to integrate the taxonomies for the adpressions and anatomically preserved floras will only cause confusion (Thomas and Cleal Citation2020); see, for instance, the discussion under Ulodendron majus in the present paper. We have never tried to promote the idea of a “preservational apartheid” when developing taxonomies for such fossils, as suggested by Bateman and DiMichele (Citation2021). Neither do we deny the significant advances that can be made in understanding the parent plants by integrating data from different types of fossils (e.g. DiMichele and Bateman Citation1996, Citation2020; DiMichele et al. Citation2013). But palaeobotanical taxonomy should not be used as a hierarchical system imposing patterns on the empirical data based on hypothetical phylogenetic models; rather, it should be regarded as a practical and therefore flexible tool for naming and classifying plant fossils (Cleal and Thomas Citation2021), which was the underlying philosophy behind our proposals made in Cleal and Thomas (Citation2010) and which became incorporated into the International Code of Nomenclature (Turland et al. Citation2018). In the case of the arborescent lycopods, combining the fossil-taxa based on adpressions and anatomically preserved remains of stems and leafy shoots does no favours to the study of the fossils in either preservations state, nor does it provide any additional clarity to our understanding of plant phylogeny.
Conclusions
Palaeobotany relies on a secure taxonomic foundation for, without it, chaos will ensue. Today, this is achieved through the use of fossil-taxa that allow different taxonomic names to be given to fossils representing different plant parts, life history stages and preservation states (Cleal and Thomas Citation2010, Citation2021); as pointed out by Bateman and DiMichele (Citation2021), fossil-taxa provide a flexible and practical tool for recording plant fossils that few palaeobotanists would want to jettison. How the fossil-taxa are circumscribed can be subjective and can often be influenced by what the palaeobotanist is trying to achieve.
For those whose primary interest is plant phylogeny, there is a temptation to merge the fossil-taxa from different plant parts and/or preservations states, with the aim of making them as near to whole-plant taxa as possible; trying to develop cladistic phylogenies will rarely be successful if based on just a small subset of the plant parts and preservation states. But this ignores the fact that taxonomy has a dual role: it is not only to classify things (in this case plant fossils, or even the fossil plants) but it is also to record things (Williams and Ebach Citation2020). For those interested in floristic or palaeoecological studies the recording aspect can be a higher priority than simple classification. For such studies, it is essential that the distribution of the plants is analysed and this is often based on fossils that in themselves would provide incomplete data for a phylogenetic analysis. Bateman and DiMichele (Citation2021) ask if “decayed pieces … found on the floors of woodlands be given a Linnaean binomial”? We would suggest that, in some cases, yes: it might not help with determining the cladistic relationships of the parent plants but it may give vital evidence as to vegetation patterns, and historical experience has shown us that this is best recorded using a Linnean-style binomial system of fossil-taxa.
How to structure the taxonomy of the fossils has to be a practical decision: what will be most useful to all palaeobotanists (not just those concerned with plant phylogeny). Ideally, the two imperatives should be accommodated; a taxonomy will be more useful in floristic and palaeoecological studies if it at least partly reflects the phylogenetic relationship of the parent plants; but equally, plant phylogeny will be better understood if we have an understanding of vegetation patterns and therefore plant fossil distribution. Ideally, the two functions of taxonomy need to be brought together to provide the most useful tool for all palaeobotanists. It must be recognised, however, that different types of fossils are used for different purposes and therefore need to be named and classified in different ways.
The classification outlined in the present paper should make it possible to identify leafy shoots of arborescent lycopsids with more confidence and allow the stratigraphical and geographical ranges of the taxa to be recorded for use in palaeogeographical and palaeoecological interpretations. The taxa are not completely “natural” in a phylogenetic sense, but neither are they totally artificial and hopefully, as more data become available (e.g. from cuticles), it may be possible to refine the fossil-taxa so they become more congruent with the taxonomic structure of the parent plants. But this is “a work in progress” and in the meantime it is vital that we have a working taxonomy to fulfil the various demands of all of today’s palaeobotanical community.
Author contributions
Both co-authors have been involved in the writing of this paper.
Data availability
All of the fossils documented in this paper are in publicly available collections, as recorded in the descriptions of each species.
Acknowledgments
The authors are grateful to the following for providing some of the images included in the paper: Sylvia Humphreys (The Great North Museum: Hancock, Newcastle upon Tyne, UK), Peter Appleton (Minera, Wrexham, UK), S. Opluštil (Charles University, Prague, Czech Republic), J. Kvaček (Národní museum, Prague, Czech Republic), Muséum national d’Histoire naturelle (Paris, France) and Chantal Montreuil (Redpath Museum, McGill University, Montreal, Canada).
Disclosure statement
The authors have had no financial interest or benefit arising from the direct applications of this research.
References
- Álvarez-Vásquez, C, RH Wagner. 2014. Lycopsida from the lower Westphalian (Middle Pennsylvanian) of the Maritime Provinces, Canada. Atlant Geol. 50:167–232. doi:10.4138/atlgeol.2014.011.
- Álvarez-Vásquez, C, J Bek, JA Knight, RH Wagner. 2018. Revision and significance of the Westphalian (Middle Pennsylvanian) arborescent lycopsid Bergeria dilatata (Lindley & Hutton) Álvarez-Vázquez & Wagner. Span J Palaeontol. 33(1):5–40. doi:10.7203/sjp.33.1.13240.
- Andrews, HN. 1955. Index of generic names of fossil plants, 1820– 1950. Bull U S Geol Surv. 1013:1–261.
- Andrews, HN, WH Murdy. 1958. Lepidophloios—and ontogeny in arborescent lycopods. Am J Bot. 45(7):552‒560. doi:10.1002/j.1537-2197.1958.tb13165.x.
- Arber, EAN. 1914. On the fossil floras of the Wyre Forest, with special reference to the geology of the coalfield and its relation to the neighbouring Coal Measure areas. Philosl Trans Roy Soc Ser B. 204:365‒445.
- Bateman, RM, WA DiMichele. 2021. Escaping the voluntary constraints of “tyre‐track” taxonomy. Taxon. 70(5):1062–1077. doi:10.1002/tax.12540.
- Bek, J, S Opluštil, J Drabkova, J Pšenička. 2015. The sub-arborescent lycopsid Omphalophloios feistmantelii (O. Feistmantel) comb. nov. emend. from the Middle Pennsylvanian of the Czech Republic. Bull Geosci. 90:227‒279.
- Bell, WA. 1938. Fossil flora of Sydney Coalfield, Nova Scotia. Canada Department of Mines and Resources. Geol Surv Mem. 215:1‒334.
- Bell, WA. 1962. Geological Survey of Canada. Flora of Pennsylvanian Pictou Group of New Brunswick. Dept Mines Tech Surv Bull. 87:1‒71.
- Bell, WA. 1966. Illustrations of Canadian fossils. Carboniferous plants of eastern Canada. Geological Survey of Canada, Paper. 66-11:1‒76.
- Boulay, N. 1876. Les terrain houiller du Nord de la France et ses végétaux fossils. Caen: Université de Caen (Thèse de geologie).
- Brack-Hanes, S, BA Thomas. 1983. A re-examination of Lepidostrobus Brongniart. Bot J Linn Soc. 86(1–2):125‒133. doi:10.1111/j.1095-8339.1983.tb00720.x.
- Brongniart, A. 1822. Sur la classification et la distribution des végétaux fossiles en général, et sur ceux des terrains de sédiment supérieur en particulier. Mémoires du Museum d’Histoire Naturelle, Paris. 8:203‒240.
- Brongniart, A. 1828. Prodrome d’une Histoire des végétaux fossiles. Paris and Strasbourg: F. G. Levrault.
- Brongniart, A. 1837. Histoire des végétaux fossiles (Vol. 2). Paris: G. Dufour & E. d’Ocagne.
- Bronn, HG. 1848. Index palaeontologicus oder übersicht der bis jetzt bekannten fossilen Organismen, Erste Abtheilung. In B. Enumerator palaeontologicus: systematische Zusammenstelung und geologische Entwicklungs-Gesetze der organischen Reiche. Stuttgart: E. Schweizerbart [in 2 volumes].
- Bureau, É. 1913‒1914. Bassin houiller de la basse Loire. Fascicule II. Description des flores fossiles. Paris: Imprimerie Nationale (Études des Gîtes Minéraux de la France). Atlas (1913), Text (1914).
- Canright, JE 1959. Fossil plants of Indiana. Indiana geological survey, Bloomington Report of Progress No. 14.
- Carruthers, W. 1869. On the structure of the stems of the Arborescent Lycopodiaceæ of the Coal Measures. Mon Microsc J. 2:177‒181.
- Chaloner, WC. 1953. On the megaspores of four species of Lepidostrobus. Ann Bot. 17(2):264–273. doi:10.1093/oxfordjournals.aob.a083350.
- Chaloner, WG. 1967. Lycophyta. In E Bureau, editor. Traité de Paléobotanique (Vol. 2). Paris: Mason at Cie; p. 435‒802.
- Chaloner, WG, B Meyer-Berthaud. 1983. Leaf and stem growth in the Lepidodendrales. Bot J Linn Soc. 86(1–2):135–148. doi:10.1111/j.1095-8339.1983.tb00721.x.
- Cleal, CJ, BA Thomas. 1994. Plant fossils of the British Coal Measures. London: Palaeontological Association. Field Guide to Fossils, 6.
- Cleal, CJ, BA Thomas. 2010. Botanical nomenclature and plant fossils. Taxon. 59(1):261‒268. doi:10.1002/tax.591024.
- Cleal, CJ, BA Thomas. 2019. Introduction to plant fossils. 2nd ed. Cambridge: Cambridge University Press.
- Cleal, CJ, BA Thomas. 2021. Naming of parts: the use of fossil-taxa in palaeobotany. Fossil Imprint. 77(1):166‒186. doi:10.37520/fi.2021.013.
- Cleal, CJ, HS Pardoe, CM Berry, B Cascales-Miñana, BAS Davis, JB Diez, MV Filipova-Marinova, T Giesecke, J Hilton, D Ivanov, et al. 2021. Palaeobotanical experiences of plant diversity in deep time. 1: how well can we identify past plant diversity in the fossil record? Palaeogeogr Palaeoclimatol Palaeoecol. 576:110481.
- Crookall, R. 1929. Coal measure plants. London: Edward Arnold & Co.
- Crookall, R 1964. Fossil plants of the Carboniferous rocks of Great Britain. (Second Section. Palaeontology. Memo Geol Surv G. B. (Palaeontol). 4(3):217–354.
- Crookall, R 1966. Fossil plants of the Carboniferous rocks of Great Britain. (Second Section. Palaeontology. Memo Geol Surv G. B. (Palaeontol). 4(4):355–571.
- Crookall, R. 1966. Fossil plants of the carboniferous rocks of Great Britain. (Second Section), Palaeontology. Memo Geol Surv G. B. (Palaeontol). 4(3):217–354.
- Daber, R. 1959. Die Mittel-Visé-Flore der Tiefbohrungen von Dorberlug-Kirchhain. Geologie Beiheft. 26:1–83.
- Dawson, JW 1863. Synopsis of the flora of the Carboniferous period in Nova Scotia. The Canadian Naturalist and Geologist and Proceedings of the Natural History Society of Montreal. 8:431‒457.
- Dawson, JW. 1866. On the conditions of the deposition of coal, more especially as illustrated by the Coal-formation of Nova Scotia and New Brunswick. Quart J Geoll Soc London. 22(1–2):95‒169. doi:10.1144/GSL.JGS.1866.022.01-02.12.
- Dawson, JW. 1868. Acadian geology the geological structure, organic remains and mineral resources of Nova Scotia, New Brunswick and Prince Edward Island. Second ed. London: Macmillan.
- DiMichele, WA. 1980. Paralycopodites Morey and Morey, from the Carboniferous of Euramerica – a reassessment of generic affinities and evolution of “Lepidodendron” brevifolium Williamson. Am J Bot. 67(10):1466‒1476. doi:10.1002/j.1537-2197.1980.tb07782.x.
- DiMichele, WA, TL Phillips. 1995. The response of hierarchically structured ecosystems to long-term climate change: a case study using tropical peat swamps of Pennsylvanian age. In S M Stanley, A J Knoll, J P Kennett, editors. Effects of past global change on life. Washington: National Academies Press; p. 134–155. Studies in Geophysics.
- DiMichele, WA, RM Bateman. 1996. The rhizomorphic lycopsids: a case‐study in paleobotanical classification. Sys Bot. 21(4):535–552. doi:10.2307/2419613.
- DiMichele, WA, SD Elrick, RM Bateman. 2013. Growth habit of the late Paleozoic rhizomorphic tree‐lycopsid family Diaphorodendraceae: phylogenetic, evolutionary, and paleoecological significance. Am J Bot. 100(8):1604‒1625. doi:10.3732/ajb.1200623.
- DiMichele, WA, RM Bateman. 2020. Better together: joint consideration of anatomy and morphology illuminates the architecture and life history of the Carboniferous arborescent lycopsid Paralycopodites. J Syst Evol. 58(6):783‒804. doi:10.1111/jse.12662.
- DiMichele, WA, RM Bateman, GW Rothwell, IA Duijnstee, SD Elrick, CV Looy. 2022. Stigmaria: a review of the anatomy, development, and functional morphology of the rootstock of the Arboreous Lycopsids. Int J Plant Sci. 183(6):493–534. doi:10.1086/7206416.
- Doweld, AB. 2013. Proposal to conserve the name Lepidophloios against Yuccites (fossil Lycopodiophyta, Lepidodendropsida). Taxon. 62:839‒840.
- Eggert, WA. 1961. The ontogeny of the Carboniferous arborescent Lycopsida. Palaeonotographica Abt B. 108:43‒92.
- Ettingshausen, C. 1854. Die Steinkohlenflora von Radnitz in Böhmen. Abhandlungen der kaiserlich-königlichen Geologischen Reichsanstalt. 2( Abt. 3, Nr. 3):1‒74.
- Feistmantel, O. 1875. Die Versteinerungen der böhmischen Kohlenablagerungen. II Abtheilung. Palaeontographica. 23:173‒190.
- Fischer, F. 1904. Nomenclatur von Lepidodendron und zur Artkritik dieser Gattung. Abhandlungen der Königlich Preußischen Geologischen Landesantalt. 39:1‒80.
- Florin, R. 1938‒1945. Die Koniferen des Oberkarbons und des unteren Perms. Palaeontographica, Abt B. 85:1‒729.
- Gastaldo, RA. 1977. A middle Pennsylvanian nodule flora from Carterville, Illinois. In R D Romans, editor. Geobotany. New York: Plenum Press; p. 133‒155.
- Geinitz, HB. 1855. Die Versteinerungen der Steinkohlenformation in Sachsen. Leipzig: W. Engelmann.
- Graham, R. 1935. An anatomical study of the leaves of the Carboniferous arborescent lycopods. Annal Bot New Ser. 49:587‒608.
- Haughton, S. 1859. Descriptions of plates XXXVIII, XXXIX, XL, XLI. Nat Hist Rev. 6:540.
- Hirmer, M. 1927. Handbuch der Paläobotanik. In I Thallophyta, editor. Bryophyta. Pteridophyta. München–Berlin: R. Oldenbourg.
- Janssen, RE. 1940. Some fossil plant types of Illinois. Illinois State Mus Sci Pap. 1:1‒124.
- Janssen, RE. 1979. Leaves and stems from fossil forests. Sprinfield IL: Illinois State Museum (Popular Science Series 1).
- Jongmans, WJ. 1909. The flora of the Dutch Carboniferous compared with that of the adjacent coalfields. In Waterschoot van der Gracht AJM. The deeper geology of the Netherlands and adjacent regions, with special reference to the latest borings in the Netherlands, Belgium and Westphalia. Mededeeling Rijksopsporing van Delfstoffen. 2:168‒247, 269‒286.
- Jongmans, WJ. 1913. Fossilium catalogus II: plantae. Pars 1 Lycopodiales I. Berlin: W. Junk; p. 1‒51.
- Jongmans, WJ. 1929. Fossilium catalogus II: plantae. Pars 15 Lycopodiales III. ‘s-Gravenhage: W. Junk; p. 54‒325.
- Josten, K-H 1991. Die Steinkohlen-Floren Nordwestdeutschlands. Fortschritte in der Geologie von Rheinland und Westfalen. 36. 1‒434.
- Kidston, R. 1885a. On the relationship of Ulodendron to L & H to Lepidodendron Stbg., Bothrodendron L. & H., Sigillaria Brgt., and Rhytidodendron Boulay. Annal Mag Natl Hist Ser. 5. 16:123‒139, 162‒179, 239‒260.
- Kidston, R. 1885b. Notes on some fossil plants collected by Mr. R. Dunlop, Airdrie, from the Lancashire Coal-field. Annal Mag Natl Hist Ser. 5. 15:473‒491.
- Kidston, R. 1886. Catalogue of the Palaeozoic plants in the department of geology and palaeontology, British Museum (Natural History). London: British Museum (Natural History.
- Kidston, R. 1889. Additional notes on some British Carboniferous lycopods. Annal Mag Natl Hist Ser. 6. 4:1–60.
- Kidston, R. 1890. The Yorkshire Carboniferous flora. Trans Yorkshire Nat Union. 14:3‒64.
- Kidston, R 1891. Notes on the Palæozoic species mentioned in Lindley and Hutton’s “Fossil Flora”. Proceedings of the Royal Physical Society of Edinburgh, 10:345‒391.
- Kidston, R. 1893a. Lepidophloios and on the British species of the genus. Trans Roy Soc Edinburgh. 37:529‒563.
- Kidston, R. 1893b. On the fossil plants of the Kilmarnock, Galston and Kilwinning coalfields. Trans Roy Soc Edinburgh. 37:307‒358.
- Kidston, R. 1894. Fossil Flora of the South Wales Coalfield and the relationship of its strata to the Somerset and Bristol Coalfield. Trans Roy Soc Edinburgh. 37:565‒614.
- Kidston, R 1902. Flora of the Carboniferous Period. Proceedings of the Yorkshire Geological and Polytechnic Society, 14:344‒370.
- Kidston, R. 1904. Carboniferous lycopods and sphenophylls. Trans Nat Hist Soc Glasgow. 6:25‒140.
- Kidston, R. 1911. Les végétaux houillers recueillis dans le Hainaut Belge. Mémoires du Musée Royal d’Histoire Naturelle de Belgique. 4(3):1‒282.
- Kidston, R. 1917. The Forest of Wyre and the Titterstone Hill Coalfields. Trans Roy Soc Edinburgh. 51(4):999‒1084. doi:10.1017/S0080456800011960.
- Koenig, C 1825, Icones fossililium dectilis. 1. Centuria prima. 1–4. 19
- Kvaček, J, J Dašková, M Libertín. 2021. Catalogue of plant fossils described in works by Kaspar M. Sternberg. Second Revised ed. Prague: National Museum (Sternbergiana; p. 1.
- Leary, RL, BA Thomas. 1989. Lepidodendron aculeatum with attached foliage: evidence of stem morphology and fossilization processes. Am J Bot. 76(2):283–288. doi:10.1002/j.1537-2197.1989.tb11311.x.
- Lebour, GA. 1878. Catalogue of the Hutton Collection of fossil plants, including a synoptical list of the chief Carboniferous species not in the collection. Newcastle-upon-Tyne: North of England Institute of Mining and Mechanical Engineers.
- Lendemer, J. 2002. A catalogue of the type specimens of the taxa erected by Leo Lesquereux in the Coal Flora (1879, 1880, 1884). Bartonia. 61:54‒80.
- Lesquereux, L. 1854. Species of fossil plants from the anthracite and bituminous coal fields of Pennsylvania, collected and described by Leo Lesquereux. J Boston Soc Nat Hist. 6:413‒431.
- Lesquereux, L. 1858. Fossil plants of the coal strata of Pennsylvania. In H D Rogers, editor. Geology of Pennsylvania (Vol. II). Edinburgh (London and Philadelphia): Blackwood and Sons, and J. B. Lippincott and Co.; p. 835‒884.
- Lesquereux, L. 1866. Report on the fossil plants of Illinois. In A H Worthen, editor. Geological survey of Illinois, Volume 2, Paleontology. Springfield IL: Geological Survey of Illinois; p. 424‒470.
- Lesquereux, L. 1870. Report on the fossil plants of Illinois. In A H Worthen, editor. Geological survey of Illinois, Volume 4, geology and paleontology, Part. 2, Section 2, paleontology of Illinois. Springfield IL: Geological Survey of Illinois; p. 377‒508.
- Lesquereux, L 1879‒1880. Description of the Coal Flora of the Carboniferous Formation in Pennsylvania and throughout the United States. Parts 1‒2. Harrisburg: geological survey of Pennsylvania ( Atlas 1879, Text 1880).
- Lesquereux, L. 1884. Description of the Coal Flora of the Carboniferous Formation in Pennsylvania and throughout the United States. Part 3. Harrisburg: Geological Survey of Pennsylvania.
- Lindley, J, W Hutton. 1831‒1833b. The fossil flora of Great Britain; or, figures and descriptions of the vegetable remains found in a fossil state in this country (Vol. 1 Part 1 ‒ 1831; Part 2 ‒ 1832; Part 3 ‒ 1833). London: John Ridgeway.
- Lindley, J, W Hutton. 1833a‒1834. The fossil flora of Great Britain; or, figures and descriptions of the vegetable remains found in a fossil state in this country (Vol. 2 Part 1 ‒ 1833; Part 2 ‒ 1834). London: John Ridgeway.
- Lindley, J, W Hutton. 1835‒1837. The fossil flora of Great Britain; or, figures and descriptions of the vegetable remains found in a fossil state in this country (Vol. 3 Part 1 ‒ 1835; Part 4 ‒ 1837). London: John Ridgeway.
- Lindsey, EM. 1915. The branching and branch shedding of Bothrodendron. Ann Bot. os-29(2):223–230. doi:10.1093/oxfordjournals.aob.a089542.
- Moore, LR, AH Cox 1943. The coal measure sequence in the Taff Valley, Glamorgan and its correlation with the Rhondda Valley sequence. Proc South Wales Inst Eng. 59:189‒304.
- Moore, R 1945. The geological sequence of the South Wales Coalfield: the ‘South Crop’ and Caerphilly Basin and its relationship with the Taff Valley sequence. Proc South Wales Inst Eng. 60:141‒252.
- Nĕmejc, F. 1934. Nekolik kritickych poznamek o Sternbergove Lepidodendron dichotomum. Rozpravy Ceske Akademie, Trida II. 44(11).
- Nĕmejc, F. 1947. The Lepidodendraceae of the coal districts of Central Bohemia. Sborník Narodního Musea v Praze. 3B(2):46‒87.
- Noé, AC. 1925. Pennsylvanian flora of Northern Illinois. Department of registration and education, division of the State geological survey. Bulletin. 52:1‒113.
- Oleksyshyn, J. 1982. Fossil plants from the Anthracite coal fields of eastern Pennsylvania. General geological report, Pennsylvanian geological survey (4th Series, Vol. 72). p. 1‒157.
- Opluštil, S, J Pšenička, M Libertín, Z Šimůnek. 2007. Vegetation patterns of Westphalian and lower Stephanian mire assemblages preserved in tuff beds of the continental basins of Czech Republic. Rev Palaeobot Palynol. 143(3–4):107‒154. doi:10.1016/j.revpalbo.2006.06.004.
- Opluštil, LM, PJB S, J Sýkorová I, J Dašková. 2009. Middle Pennsylvanian pioneer plant assemblage buried in situ by volcanic ash-fall, central Bohemia, Czech Republic. Rev Palaeobot Palynol. 155:204‒233.
- Opluštil, S, J Pšenička, M Libertín, A Bashforth, Z Šimůnek. 2009a. A Middle Pennsylvanian (Bolsovian) peat-forming forest preserved in situ in volcanic ash of the Whetstone Horizon in the Radnice Basin, Czech Republic. Rev Palaeobot Palynol. 155(3–4):234‒274. doi:10.1016/j.revpalbo.2009.03.002.
- Opluštil, S, J Pšenička, M Libertín, J Bek, J Daskova, Z Šimůnek, J Drábková. 2009b. Composition and structure of an in situ Middle Pennsylvanian peat-forming plant assemblage buried in volcanic ash, Radnice Basin (Czech Republic). Palaios. 24(11):726‒746. doi:10.2110/palo.2008.p08-128r.
- Opluštil, S. 2010. Contribution to knowledge on ontogenetic developmental stages of Lepidodendron mannebachense Presl, 1838. Bull Geosci. 85:303–316. doi:10.3140/bull.geosci.1180.
- Opluštil, S, J Pšenička, J Bek, J Wang, Z Feng, M Libertín, Z Šimůnek, J Bureš, J Drabkova. 2014. T0 peat-forming plant assemblage preserved in growth position by volcanic ash-fall: a case study from the Middle Pennsylvanian of the Czech Republic. Bull Geosci. 89:773‒818.
- Opluštil, S, J Pšenička, Z Šimůnek, M Libertín. 2016. Floras of clastic and peat-forming Pennsylvanian wetlands: are they different? A case study from the Upper Radnice Coal (late Duckmantian), Kladno Coalfield, Czech Republic. Span J Palaeontol. 31:145‒180.
- Pal, PK, AK Ghosh. 1990. Proposal to conserve Lycopodites Lindley and Hutton against Lycopodites Brongniart (Fossiles). Taxon. 39:129–130.
- Pearson, HL. 1986. Structure and taxonomy of the Carboniferous lycopsid Anabathra. Bulletin of the British Museum (Natural History. Geol Ser. 40:265‒292.
- Popa, ME. 2016. Lepidophloios acerosus Lindley and Hutton 1831 in the Carboniferous Cucuiova Formation, “Iron Gates” Natural Park (Banat, Romania). Transylvanian Rev Sys Ecol Res. 16:1‒10.
- Remy, W, R Remy. 1959. Pflanzenfossilien. Berlin: Akademie-Verlag.
- Renier, A. 1926. La morphologie gėnèrale des Ulodendron. Comptes rendus hebdomadaires des sėances de l’Acadėmie des sciences. Paris. 182:408‒410.
- Rhode, JG. 1823. Beiträge zur Pflanzenkunde der Vorwelt: nach Abdrücken im Kohlenschiefer und Sandstein aus schlesischen Steinkohlenwerken. Breslau.
- Schlotheim von, EF. 1820. Die Petrefactenkunde auf ihrem jetzigen Standpunkte durch die Beschreibung seiner Sammlung versteinerter und fossiler Überreste des Thier- und Pflanzenreichs der Vorwelt erläutert. Gotha: Beckerschen Buchhandlung.
- Sternberg, KM. 1820‒1821. Versuch einer geognostisch-botanischen Darstellung der Flora der Vorwelt, Band 1 (Part 1 ‒ 1820; Part 2 ‒ 1821). Leipzig: F. Fleischer.
- Sternberg, KM. 1825. Versuch einer geognostisch-botanischen Darstellung der Flora der Vorwelt, Band 1 (Part 4, Tentamen). Regensburg: E. Brenck’s Witwe.
- Sternberg, KM. 1838. Versuch einer geognostisch-botanischen Darstellung der Flora der Vorwelt, Band 2 (Parts 7‒8). Prague: G. Hässe und Söhne.
- Sterzel, JT. 1907. Die Karbon- und Rotliegenfloren im Grossherzogtum Baden. Mitteilungen der Grossherzoglich Badischen Geologischen Landesanstalt. 5:347‒892.
- Stockmans, F, Y Willière. 1953. Végétaux namuriens de la Belgique. Association pour étude de la Paléontologie et de la stratigraphie Houillères. 13:1‒382.
- Strullu-Derrien, C, CJ Cleal, C Ducassou, ART Spencer, E Stolle, VO Leshyk. 2021. A rare late Mississippian flora from NorthWestern Europe (Maine-et-Loire Coalfield, Pays de la Loire, France). Rev Palaeobot Palynol. 285:104359. doi:10.1016/j.revpalbo.2020.104359.
- Thomas, BA. 1966. The cuticle of the lepidodendroid stem. New Phytol. 63:296‒503.
- Thomas, BA. 1967a. The cuticle of two species of Bothrodendron [Lycopsida: lepidodendrales]. J Nat Hist. 1(1):53‒60. doi:10.1080/00222936700770621.
- Thomas, BA. 1967b. Ulodendron: Lindley and Hutton and its Cuticle. Ann Bot. 31(4):775‒782. doi:10.1093/oxfordjournals.aob.a084181.
- Thomas, BA. 1968. The carboniferous fossil lycopod Ulodendron landsburgii (Kidston) comb. nov. J Nat Hist. 2(3):425‒428. doi:10.1080/00222936800770401.
- Thomas, BA. 1970. Epidermal studies in the interpretation of Lepidodendron species. Palaeontology. 13:145‒173.
- Thomas, BA. 1974. The lepidodendroid stoma. Palaeontology. 17:525‒539.
- Thomas, BA, J Watson. 1976. A rediscovered 114-foot Lepidodendron from Bolton, Lancashire. Geol J. 11:15‒20.
- Thomas, BA. 1977. Epidermal studies in the interpretation of Lepidophloios species. Palaeontology. 20:273‒293.
- Thomas, BA. 1978. Carboniferous Lepidodendraceae and Lepidocarpaceae. Bot Rev. 44(3):321‒364. doi:10.1007/BF02957853.
- Thomas, BA, SV Meyen. 1984a. A reappraisal of the Lower Carboniferous lepidophyte Eskdalia Kidston. Palaeontology. 27:707‒718.
- Thomas, BA, SV Meyen. 1984b. Tunguskadendron borkii: gen. et sp. nov. A new Angaran lepidophyte. Palaeontographica Abt B. 19:121‒126.
- Thomas, BA, CJ Cleal. 1999. Abscission in the fossil record. In M R Kurmann, A R Hemsley, editors. Evolution of plant architecture. Kew: Royal Botanic Gardens; p. 183‒203.
- Thomas, BA, Y Tenchov. 2004. The Upper Westphalian lycopods of the Dobrudzha Coalfield (Bulgaria) and a comparison with those of Southern Britain and Cape Breton, Canada. Geol Balcan. 35:105‒110.
- Thomas, BA. 2007. Phytogeography of Asturian (Westphalian D) lycopods throughout the belt of Euramerican coalfields. Geol Mag. 166:457‒463.
- Thomas, BA, E Zodrow, CJ Cleal. 2010. Leafy branches of Bothrodendron punctatum from the Westphalian D (Asturian) of Nova Scotia, Canada. Atlant Geol. 46:1‒6. doi:10.4138/atlgeol.2010.001.
- Thomas, BA, LJ Seyfullah. 2015a. Stigmaria Brongniart: a new specimen from Duckmantian (Lower Pennsylvanian) Brymbo (Wrexham, North Wales) together with a review of known casts and how they were preserved. Geol Mag. 152(5):858‒870. doi:10.1017/S0016756815000035.
- Thomas, BA, LJ Seyfullah. 2015b. A new look at Lepidodendron ophiurus Brongniart and the recognition of L. wingfieldense sp. nov. (Fleminigitaceae, Lepidodendrales) from the Langsettian (Bashkirian) of Derbyshire, East Midlands, UK. Palaeontographica, Abt B. 292(1–3):23‒32. doi:10.1127/palb/292/2015/23.
- Thomas, BA, CJ Cleal. 2018. Arborescent lycophyte growth in the late Carboniferous coal swamps. New Phytol. 218(3):885‒890. doi:10.1111/nph.14903.
- Thomas, BA, NA Judd, LJ Seyfullah. 2019. A comparison of Asturian lepidodendroid lycopods from the USA and the European Variscan Font. Geobios. 56:31‒48. doi:10.1016/j.geobios.2019.07.002.
- Thomas, BA, P Appleton, CJ Cleal, LJ Seyfullah. 2019. The distribution of plant fossils and their palaeoecology in Duckmantian (Bashkirian, Lower Pennsylvanian) strata at Brymbo, North Wales, UK. Geol J. 55:3179‒3207.
- Thomas, BA, CJ Cleal. 2020. The nomenclature of fossil-taxa representing different preservational states: Lepidodendron as a case-study. Taxon. 69(5):1061‒1092. doi:10.1002/tax.12291.
- Unger, F. 1850. Genera et Species Plantarum Fossilien. Wien: Wilhelmum Bauműller.
- van Amerom, HWJ. 1975. Biostratigrafie van het Carboon in Nederland. In W Zagwijn, C Staalduinen, editors. Toelichting bij Geologische Overzichtskaarten van Nederland. Haarlem: Rijks Geologische Dienst; p. 123‒134.
- von Röhl, E. 1868. Fossile Flora der Steinkohlenformation Westphalens, einschliesslich Piesberg bei Osnabrűck. Palaeontogtaphica. 18:1‒191.
- Wagner, RH, C Álvarez-Vázquez. 2015. A coastal forest swamp dominated by Omphalophloios C. D. White, in the Autunian (uppermost Stephanian) of Puertollano, south-central Spain. Palaeontographica, Abt B. 292(1–3):33‒77. doi:10.1127/palb/292/2015/33.
- Walton, J. 1935. XIII.—Scottish Lower Carboniferous Plants: the Fossil Hollow Trees of Arran and their Branches (Lepidophloios wünschianus Carruthers). Trans Roy Soc Edinburgh. 58(2):313‒337. doi:10.1017/S0080456800018962.
- Wang, Q. 2007a-1772. Proposal to conserve the name Microsorium (Polypodiaceae) with that spelling. Taxon. 56(2):268–269. doi:10.1002/tax.562037.
- Wang, Q. 2007b. On the correct name for arborescent lycopsid Lepidophloios (Lepidodendraceae). J Syst Evol. 45:415‒420.
- Weiss, E. 1893. Die Sigillarien der preussischen Steinkohlen- und Rothliegenden-Gebiete. II. Die Gruppe der Subsigillarien. Abhandlungen der Königlich Preussischen geologischen Landesanstalt, Neue Folge. 2:1‒255.
- White, D. 1898. Omphalophloios, a new lepidodendroid type. Bull Geol Soc Am. 9:329‒342.
- Wiersema JH, TNJ, FR Barrie, W Greuter, DL Hawksworth, PS Herendeen, S Knapp, WH Kusber, DZ Li, K Marhold, et al. eds. 2018. International code of nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the nineteenth international botanical congress Shenzhen, China, July 2017. Glashütten: Koeltz Botanical Books (Regnum Vegetabile 159).
- Williams, DM, ME Ebach. 2020. Cladistics. A guide to biological classification. 3rd ed. Cambridge: Cambridge University Press.
- Williamson, WC. 1872. On the organization of the fossil plants of the coal-measures—Part II. Lycopodiaceæ: Lepidodendra and Sigillariæ. Philos Trans Roy Soc London. 162:197‒240.
- Witham, HTM. 1833. The internal structure of fossil vegetables found in the Carboniferous and Oolitic deposits of Great Britain. Described and Illustrat ed. Edinburgh: A & C Black.
- Wood, JM. 1968. The Stanley Cemetery Flora (Early Pennsylvanian) of Greene County, Indiana. Ind Geoll Surv Bull. 29:1–73.
- Zeiller, R. 1879‒1880. Végétaux fossiles du terrain houiller de la France. Paris: Imprimerie Nationale. Atlas (1879), Text (1880).
- Zeiller, R. 1885. Présentation d’une brochure de M, Kidston sur les Ulodendron, et Observations sur les genres Ulodendron et Bothrodendron. Bulletin de la Société Géologique de France, Sér. 6. 14:168‒182.
- Zeiller, R. 1886. Bassin houiller de Valenciennes, description de la flore fossile (Atlas). Paris: Imprimerie Nationale (Études des Gîtes Minéraux de la France).
- Zeiller, R. 1888. Bassin houiller de Valenciennes, description de la flore fossile (Text). Paris: Imprimerie Nationale (Études des Gîtes Minéraux de la France).
- Zodrow, EL, K McCandlish. 1980. Upper Carboniferous fossil flora of Nova Scotia. In the collections of the Nova Scotia Museum, with special reference to the Sydney Coalfield. Halifax N.S.: Nova Scotia Museum.
- Zodrow, EL, M Mastalerz, R Helleur. 2012. Lepidodendron dawsonii: functional groups and pyrolysates of compression and fossilized-cuticle (Late Asturian, Canada). Geologia Croatica. 65(3):367‒374. doi:10.4154/GC.2012.26.
