ABSTRACT
Objective: Since the 1970s, research and applications on flap and muscle flap had solved many problems in microsurgical reconstruction. However, the traditional flap design is completely dependent on two-dimensional (2D) images. The purpose of this study was to discuss the methods in the visualization of saphenous artery flap by digitalized technique and its applications by digitalized technique.
Methods: Two adult fresh cadaver specimens, one male and one female, were subject to radiographic computerized tomography (CT) scanning before and after perfused with lead oxide–gelatine mixture, whose collimation are 0.625 mm (120 kV, 110 mA, 512 × 512 matrix). Through Amira 5.4.1 software, the 2D images in DICOM format were transformed into the 3D models of the entire region. The structures of saphenous artery were observed and the digitized visible models of saphenous artery flap were established through three-dimensional (3D) computerized reconstructions methods from these data using Amira 5.4.1 software. Next six cases of soft-tissue defects of the tibia region, involving the exposure bones underwent contrast-enhanced CT angiography of lower limbs utilizing a 64-row multi-slice spiral CT after median cubital vein injection with Ultravist (3.5 ml/s). 2D images from these data in DICOM format were transformed into computer. The structures of saphenous artery flap were observed and measured using Amira 5.4.1 software. Then, all cases were treated by saphenous artery flap.
Results: The 3D reconstructed visible models established from these datasets perfectly displayed the saphenous artery flap anatomy. In six cases, the main trunk and branched of the blood vessels in the designed flap were consistent with the surgical findings. The starting point of the saphenous artery to the average distance of the knee clearance were 119.2 ± 9.6 mm, the average diameter of the saphenous artery from the starting point were 1.5 ± 0.3 mm. The range of flap was 8.0 × 5.0 cm to 20.0 × 8.0 cm. All flaps survived well. After 8–24 months’ follow-up the knee flexion was 120–140°, the straight 0–10°. There was no case appeared incision infection.
Conclusions: The preoperative use of 3D digitalized virtual planning for the saphenous artery flap improves the surgical accuracy, decreases the operation time and increases the survival rate of the flap.
Introduction
Anatomy and clinical practice-advanced flap research and application address the challenging problem of microsurgical reconstruction in many new ways. In 1936, Michel Salmon used lead oxide–gelatin corpse perfusion technology to reveal the vascular structures in the skin. Since then, the technology has been widely used and improved as the gold standard for vascular visualization.[Citation1–5] Recently, the development of digitalized techniques and diagnostic imaging techniques in the field of microsurgery has made it possible to apply three-dimensional (3D) reconstruction to observe the stereoscopic anatomy of the flap.[Citation6] The flap blood supply can be visualized stereoscopically preoperatively, thus allowing the best design of the flap, the best surgical approach and the most suitable therapy protocol. 3D reconstructed images have been used in surgical reconstruction with latissimus dorsi muscular flaps, anterolateral thigh flaps and dorsalis pedis flaps.[Citation7–10] Complete 3D image of flap blood supply can be built by perfusion specimens and digital technology, which allows clinicians to intuitively understand the relationship between blood vessels, skin and muscle, as well as a complete vascular morphology. This article explored the role of digital 3D reconstruction technique in saphenous artery flap anatomy and visualization.
Materials and methods
CT scan and 3D reconstruction of fresh corpse perfusion specimens
Two fresh adult cadavers from donation, one each for men and women with no obvious peripheral vascular lesions and scarring were involved. The improved lead oxide–gelatin perfusion method described by Tang et al. [Citation11] was used. Before and after perfusion, continuous row spiral CT scans were performed: voltage 120 kV, slice thickness 0.625 mm, matrix of 512 × 512. DICOM format images were obtained and imported into Amira 5.4.1 software (Visualization Sciences Group, Merignac, France) for 3D surface reconstruction of the thigh vessels, femur and skin. Thigh vessels were also processed by volume reconstruction and image registration to visualize saphenous artery and build saphenous artery flap.
Clinical application
From June 2010 to August 2011, six cases of tibial skin defects and bone exposed patients were treated with saphenous artery flap, including three males and three females, aged 18–32 years, mean 25.6 years were involved. No lower limb deformities and trauma of the knee were found. The experiment protocol was approved by Institutional Review Board and all patients signed informed consent. After injection of non-ionic contrast agent Ultravist 370 (Guangzhou Pharmaceuticals Corporation, Guangzhou, China) through median cubital vein, consecutive spiral CT scan were performed using GE Light Speed 64 of the two legs at straight. The main CT scanning parameters were as follows: 120 kV, 200 mA; slice thickness 2.5 cm (final reconstructed images were partitioned into 0.625 mm thick images). Serial data from the thigh to the tibial plateau regions in DICOM format were imported to a personal computer. 3D reconstruction was then performed using Amira 5.4.1 software. Continuous observe the thigh and knee vessel, particularly the knee descending artery and the saphenous artery. The 3D reconstruction consisted of (i) tracing the contours of the anatomical structures to be reconstructed; (ii) adjustment by geometrical alignment of the contours of stacked points and (iii) modeling of the surfaces by meshing the frame work of the points transformed into polygons (wire-framed object) and smoothing the contours of the object reconstructed from points (surface rendering). The anatomical location of blood vessels, skin and muscles was observed to determine the position of the flap. The 3D Lasso tool was used to select areas in the image based on the size and shape of the defects. Double-blind methods were used to measure the perpendicular distance from the start of saphenous artery to the gap of the knee joint. Retrograde island flap design was adopted when taking 3D reconstruction results as reference. A line parallel to the longitudinal axis of the lower limbs were drawn from the middle of medial knee joint to the middle of medial calf, defined as the axis of the flap. Flap was designed within the range of 5.0 cm from both sides of the line and 15.0 cm above and 8.0 cm below the knee. Completely remove the scar tissue necrosis and granulation tissue inflammation. Based on the results of the 3D surface reconstruction of the saphenous artery, flap in a retrograde fashion was done. The dissection was carried under the deep fascia until the perforating branches of the saphenous artery were found. The flap was retrogradely transferred to the recipient site. Based on the tension of the distal pedicle of the flap, the flap was transferred by opening the intervening skin. If the tension in the donor site was small, the flap was directly sutured. If the tension in the donor site was large, the defects were repaired with autologous thick pieces.
Results
Digital reconstruction
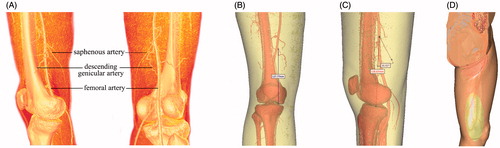
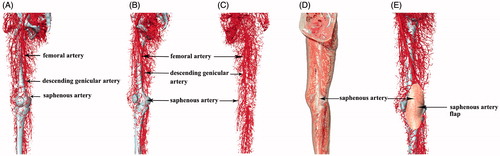
Digital reconstruction showed the main components of the saphenous artery and its surrounding. Various anatomical structures can be clearly visualized in the reconstructed images ( and ), especially the saphenous artery and its projection. The morphological features were clearly displayed by registration of the 3D volume and surface reconstruction. The saphenous artery was reconstructed and observed at different angles. The reconstructed structure of the skin and arteries and their anatomical relationship were clearly observed with strong physical sensation.
Figure 1. CT scanning image reconstruction of saphenous artery and its surrounding blood vessels in perfusion specimen. (A, B and C) Surface reconstruction femoral artery, descending genicular artery and saphenous artery. (D and E) The projection on the body surface and the scope of the saphenous artery and saphenous artery flap.
Clinical application
All the flaps were designed before the operation according to the reconstructed arteries. Therefore, the size and shape of the flap was known before the operation. The flap displayed in six patients was main branch and perforator vessels. Average distance between saphenous artery starting point and knee joint gap was 119.2 ± 9.6 mm. Average diameter of the saphenous artery starting point was 1.5 ± 0.3 mm. The flap scope ranged from 8.0 × 5.0 to 20.0 × 8.0 cm. The distribution of blood vessels identified by intra operative exploration matched the reconstructed images. Flap and skin graft donor sites survived and flap survival time were 2–4 weeks, with an average of 2.8 weeks. The six patients were followed up for 8–24 months, averaged 18 months. Knee flexion was 120–140°, and knee straight was 0–10°.
Typical case
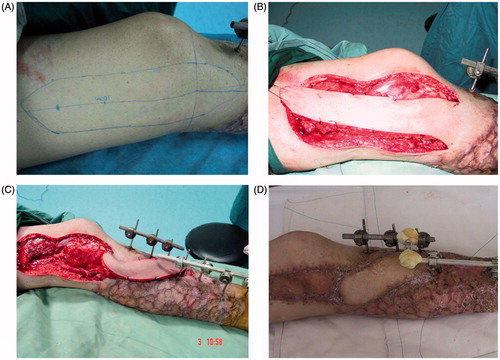
Sixty-five years old male, whose left leg, was crushed. Injury was treated 2 h after. The left leg had annular skin defects from the ankle to the tibial tuberosity, 10.0 cm defect from the tibialis anterior muscle and to the tibialis anterior blood vessels, comminuted fracture of the tibial lower-middle section. The fracture directly connected with outside, setting off widespread skin bruising. After 4 weeks of the first operation, the majority wound part of shank healed, with only the front of the tibia bone exposed. Retrograde descending saphenous artery flap repair was performed according 3D virtual design. Flap cut range was 15.0 × 8.0 cm. After 2 weeks, flap survived completely ().
Discussion
The saphenous artery flap is a fasciocutaneous flap generally used for knee and upper third of the leg coverage. Due to various descriptions of the saphenous flap, such as venous, sensory and free flap, the origin and distributing characteristics of the saphenous artery are important for plastic surgeons.[Citation12,Citation13] In general, the descending genicular artery arises from the superficial femoral artery just before it passes through the opening in the tendon of the adductor magnus. After descending 0.5–2.0 cm, the descending genicular artery is divided into the musculoarticular branch and the saphenous branch.[Citation14]
Since the 1970s, research and applications on flap and muscle flap had solved many problems in microsurgical reconstruction. However, the traditional flap design is completely dependent on 2D images on textbook and autopsy. A question remained if we can reveal the flap in a 3D anatomical, visualized, dynamic way to enhance the understanding between adjacent blood vessels, muscles, bones and skin. CT-based digital technology has solved this problem. 3D images and models can be used in clinical anatomy teaching, preoperative design and virtual operation simulation in the future. Currently through 3D reconstruction technology, we can rebuild the main flap constitutes – vessels.[Citation10,Citation11] 3D reconstruction technology allows for greater accuracy in locating perforators and identifying their surrounding landmarks. The three-dimensional model segmentation and editing tools enable data manipulation and allow the selection of specific tissue types (e.g. bone, fat, skin and vessels). Once an area of interest is separated, it can be visualized in three dimensions. These features allow surgeons to improve their flap design by mapping the distribution of surrounding vessels and by planning and simulating surgical procedures. In comparison with two-dimensional angiography, 3D images have fewer artifacts because of the ability to digitally edit them in a 3D fashion.[Citation11,Citation15] Since gelatin–lead oxide technology can provide high-quality vascular imaging, vessels larger than 0.3 mm can be displayed as a good basis for the study of saphenous artery, providing a clear and realistic flap vessels network, including connection and distribution. In this study by CT scan of the gelatin–lead oxide perfusion model, saphenous artery matched well with the major blood vessels of the lower extremity. A densely matched network of blood vessels was formed, including the articular branch of the femoral artery, the branch of the popliteal artery, the anterior tibial artery, etc., which provided a reliable theoretical basis for saphenous artery flap design. Through subject specific 3D image visualization technologies, the traditional surgical model can be changed to “observation, exposure and surgery”. Before exposure of the operation site, using the patient’s individualized computer image reconstruction, 3D model can be manipulated in the surgical planning software. Advantages include as: in-depth observation of the surgical site, exact planning of the path and scope of the surgery, and avoiding secondary injury of the vascular structures in the surgery. Better survivorship of the flap can be ensured. 3D digitalized images of the saphenous artery flap exhibit personalized features, and can be stereoscopically and dynamically visualized in multiple angles. 3D reconstructed images can accurately locate the source and pattern of blood supply for the flap, thus improve insights clinical application in the field of microsurgery. The preoperative use of 3D digitalized virtual planning for saphenous artery flaps improves the effectiveness and safety of surgical repair of soft-tissue defects.
Locate the cutaneous branch of saphenous artery muscular is the key to the design of the flap. The current practice use preoperative Doppler probe to locate cutaneous branch piercing point and clarify its position, path, caliber and flow conditions. Angiography can more intuitively display the saphenous artery starting point and path. Due to the limitations of imaging techniques, perforator and vascular network cannot be shown, unlike gelatin–lead oxide perfusion models. Angiography can ensure the starting point of the saphenous artery flap and aid in the flap design. This study showed that the artery and saphenous shape from preoperative 3D reconstruction matched well to intra-operative clinical findings. However, preoperative flap design can only provide a general idea, not the precise data. The range of flap still requires the surgeon’s experience.
The study used digital technology to reconstruct and visualize saphenous artery flap and successfully applied the results in clinical. Flap survived with satisfaction. However due to the limitation of angiographic imaging techniques, small branch cannot be displayed. This digital reconstruction approach can only provide a general resection range. In addition, the study selected cases with only retrograde saphenous artery flap, and was limited to a small number. We report the results of only a preliminary application of digital technology in the saphenous artery flap. Through digital reconstruction and measurement, the starting point of saphenous artery, path and generally distribution can be relatively accurately positioned. This provided a new design approach for clinical application.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
This work was supported by Nature Science Foundation of Inner Mongolia Autonomous Region of China [2009MS1103].
References
- Taylor GI, Gianoutsos MP, Morris SF. The neurovascular territories of the skin and muscles: anatomic study and clinical implications. Plast Reconstr Surg. 1994;94:1–36.
- Rees MJ, Taylor GI. A simplified lead oxide cadaver injection technique. Plast Reconstr Surg. 1986;77:141–145.
- Wu WC, Chang YP, So YC, et al. The combined use of flaps based on the subscapular vascular system for limb reconstruction. Brit J Plast Surg. 1997;50:73–80.
- Angrigiani C, Grilli D, Siebert J. Latissimus dorsi musculocutaeous flap without muscle. Plast Reconstr Surg. 1995;96:1608–1614.
- Heitmann C, Guerra A, Metzinger SW, et al. The thoracodorsal artery perforator flap: anatomic basis and clinical application. Ann Plast Surg. 2003;51:23–29.
- Park YS, Sung KS. Surgical reconstruction of chronic Achilles tendon ruptures using various methods. Orthopedics. 2012;35:e213–e218.
- Colohan S, Wong C, Lakhiani C, et al. The free descending branch muscle-sparing latissimus dorsi flap: vascular anatomy and clinical applications. Plast Reconstr Surg. 2012;130:e776–e787.
- Heo C, Eun S, Bae R, Minn K. Distally based anterolateral-thigh (ALT) flap with the aid of multidetector computed tomography. J Plast Reconstr Aesthet Surg. 2010;63:e465–e468.
- Zhang YZ, Li YB, Jiang YH, et al. Three-dimensional reconstructive methods in the visualization of anterolateral thigh flap. Surg Radiol Anat. 2008;30:77–81.
- Zhang YZ, Li YB, Tang ML, et al. Application of three-dimensional digitalized reconstruction of an anterolateral thigh flap and an arterial dorsalis pedis flap. Microsurgery. 2007;27:553–559.
- Tang M, Geddes CR, Yang D, et al. Modified lead oxide-gelatin injection technique for vascular studies. J Clin Anat. 2002;1:73–78.
- Gocmen-Mas N, Aksu F, Edizer M, et al. The arterial anatomy of the saphenous flap: a cadaveric study. Folia Morphol (Warsz). 2012;71:10–14.
- Nenad T, Reiner W, Michael S, et al. Saphenous perforator flap for reconstructive surgery in the lower leg and the foot: a clinical study of 50 patients with posttraumatic osteomyelitis. J Trauma. 2010;68:1200–1207.
- Lee SH, Choi TH, Kim SW, et al. An anatomical study of the saphenous nerve, artery, and artery perforators within the thigh using cadaveric dissection. Ann Plast Surg. 2011;67:413–415.
- Tang M, Mao Y, Almutairi K, et al. Three-dimensional analysis of perforators of the posterior leg. Plast Reconstr Surg. 2009;123:1729–1738.