Abstract
Acetabular fractures have always been very challenging for orthopedic surgeons; therefore, appropriate preoperative evaluation and planning are particularly important. This study aimed to explore the application methods and clinical value of preoperative computer simulation (PCS) in treating pelvic and acetabular fractures. Spiral computed tomography (CT) was performed on 13 patients with pelvic and acetabular fractures, and Digital Imaging and Communications in Medicine (DICOM) data were then input into Mimics software to reconstruct three-dimensional (3D) models of actual pelvic and acetabular fractures for preoperative simulative reduction and fixation, and to simulate each surgical procedure. The times needed for virtual surgical modeling and reduction and fixation were also recorded. The average fracture-modeling time was 45 min (30–70 min), and the average time for bone reduction and fixation was 28 min (16–45 min). Among the surgical approaches planned for these 13 patients, 12 were finally adopted; 12 cases used the simulated surgical fixation, and only 1 case used a partial planned fixation method. PCS can provide accurate surgical plans and data support for actual surgeries.
Introduction
The management of pelvic and acetabular fractures is usually highly complex, and it is a challenge for surgeons, requiring the selection of a surgical approach, full understanding of anatomic conditions, fracture reduction, and internal fixation methods.[Citation1,Citation2] The goal of surgical treatment of a comminuted pelvic fracture is to maintain pelvic anatomic shape and restore biomechanical characteristics.[Citation3] The surgical treatment of acetabular fractures should follow the basic principles used for intra-articular fractures: anatomical reduction of the articular surface and firm internal fixation, in order to facilitate early postoperative functional exercise and reduce the possibility of postoperative traumatic arthritis.[Citation4,Citation5] The treatment principles of pelvic and acetabular fractures might seem simple, but this surgery often involves a number of specific and difficult issues. Although the functional anatomy of the pelvis and acetabulum has been well studied, and three-dimensional (3D) computed tomography (CT) has improved the diagnostic capabilities of imaging, thorough understanding of the fracture line and fragments remains problematic. The surgical approach is particularly important in pelvic and acetabular fractures, but the correct choice of surgical approach is another question. The reduction of fracture fragments is usually of high importance, as these play key roles in the biomechanical functions of the pelvis and acetabulum. When dealing with large bones, the limitations of incision size can cause small bone fragments to remain concealed, resulting in difficulty in completely and precisely controlling bone reduction during surgery. After reduction, the problem of fixation must be dealt with; for example, the plate must be precisely shaped on three planes to be suitable for the pelvic forms of different individuals. Furthermore, the key screws must be precisely placed, and screw breakthrough on the articular surface must be avoided.
In summary, strict preoperative planning is critical in pelvic and acetabular surgery. The Virtual Operation Planning in Orthopaedic Surgery (VIRTOPS) software can improve surgical precision,[Citation6–8] and its use has been reported for pelvic tumors, individual prosthesis design, and maxillofacial surgery,[Citation9–14] but rarely in pelvic and acetabular fractures. We used Mimics software (Materialise interactive medical image control system, Materialise Co., Belgium) for VIRTOPS in treating pelvic and acetabular fractures; the purpose of our study was to explore the application methods and clinical value of VIRTOPS in treating pelvic and acetabular fractures.
Materials and methods
Patients and data acquisition
In all, 13 patients with pelvic and acetabular fractures were included in this study; based on AO classification, three cases were type A, six were type B, and four were type C; all patients underwent preoperative radiography and spiral computed tomography (Sensation 64, Siemens Medical Solutions, Germany). This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Southern Medical University. Written informed consent was obtained from all participants.
Establishment of digital model
The original patient data were input with the Digital Imaging and Communications in Medicine (DICOM) format, and the thickness of the fault was set as 1.5 mm to obtain a vivid virtual pelvis. The simulation used a semiautomatic plus manual segmentation model: first, the initial value of bone density was determined; second, the bone densities were automatically segmented; and third, the data were checked and repaired using a manual segmentation method, particularly for those bone fragments that are too small to be automatically segmented. Thus, each bone chip would eventually appear as a separate object, and be assigned a different color.
Simulation and design of surgical procedures
All virtual objects (bone blocks) could independently and freely move, rotate, and be reduced and removed in a 3D plane, in order to observe each bone block in the model, and to clarify the spatial relationships of each block. The intraoperative anatomical landmarks and reduction sequences of every block can be determined, as can the methods of pre-fixation and final strengthening-fixation of every block; meanwhile, the intraoperative incisions and fixation methods can also be determined using the model. Once the reduction has succeeded, certain suitable fixation materials can thus be chosen. Based on a successfully reduced pelvis, the contours of the plate can be outlined; then, by rotating the pelvic digital model and changing its transparency, controllable screw length and directions and all simulated surgical procedures can be recorded and printed.
Main observation indexes
The times needed for creation of a patient’s individual digital model, the reduction of the virtual fracture, and the fixation operation were measured. All patients underwent repeat CT within 2–4 days after surgery. The reduction of the articular surface was assessed by Matta’s criteria for anatomic reduction: optimal displacement of the articular surface is less than 1 mm, 2–3 mm is satisfactory, and more than 3 mm is unsatisfactory.
Virtual surgical procedure
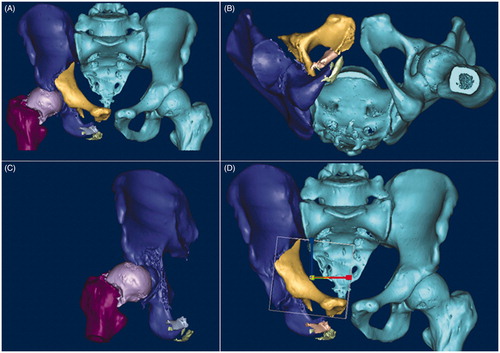
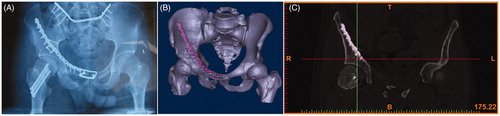
The procedure is presented as a case study. A typical case was used to demonstrate the entire procedure: A 26-year-old man sustained multiple injuries due to a fall, and underwent surgery 15 days later. The patient had a left acetabular fracture, femoral neck fracture, and dislocation of the pubic symphysis and sacroiliac joints. The CT data in DICOM format were imported into Mimics (Materialise, Belgium) on a personal computer. The appropriate threshold (in Hounsfield units) that specified the desired densities defining bone was determined first. The next step was segmentation, which had to be performed manually for all slices in all three planes. Different colors were assigned to different fracture fragments. After the segmentation process, each fracture fragment became a separate object. Then, a pelvic model with fractured acetabulum was built for the simulation (). The pelvis could be moved in all directions during the virtual operation, and key bone fragments could be identified. After 3D reconstruction, the reduction of the fracture was performed, as bone fragments could be moved and rotated in all three planes (). Finally, the reduced model was saved in the .stl format. Bone fragments could be removed to observe spatial relationships, key bone fragments could be identified (), and fragments could be rotated and moved for the reduction (). On the post-reduction model, the surgeon could choose the appropriate internal implant. The key bone fragments of the anterior column were first pre-fixed using two bow-teeth screws (developed by our group, and produced by Seemine SMA Co, Lanzhou, China) to ensure the efficacy of reduction, then reinforced with one reconstruction plate (). The dislocation of the sacroiliac joint in this patient was treated using an ilium-iliac plate-fixation method, which has been studied by our group, but has not been reported yet (). The acetabular posterior horn fracture in this patient exhibited no significant shift, and no internal fixation was performed because it was not a weight-bearing area.
Results
Evaluation of software operation time
Subdivision of the fracture was expected to be the most time-consuming in preoperative planning. Six patients with more bone fracture fragments and three with severe impaction underwent manual subdivision. The average time used for splitting the bone fractures from the entire pelvis and extracting the articular surface model was 45 min (30–70 min), and the CAD module that came with the software allowed direct and intuitive manipulation of the fracture fragments; after training, the average time for fracture reduction and fixation was 28 min (16–45 min).
Evaluation of postoperative effects
In 13 cases with planned surgical approaches, 12 approaches were completely adopted. An ilioinguinal approach was planned in five patients, and adopted in all five patients. Of six cases with a planned Kocher–Langenbeck approach, five cases were adopted. One case of transverse acetabular fracture added an anterior ilioinguinal approach for a difficult intraoperative reduction. The Kocher–Langenbeck plus ilioinguinal approach was planned for two cases, but was finally adopted in three cases.
The simulated surgical fixation methods were completely applied in 12 cases, and 1 case partially used the planned fixation method; in 10 cases, the same number of screws was implanted as in preoperative planning, and the length of the screws was the same as in preoperative planning in 8 cases; 11 cases used the same number of fixation plates, and the positions of the fixation plates in all 11 cases were the same as in preoperative planning.
According to Matta’s criteria, postoperative CT showed that the articular surface in six cases (46%) exhibited anatomic reduction, and the effects in seven cases (54%) were satisfactory; no screw penetrated into the joint cavity, and no serious complications such as deep infection and fixation failure occurred; however, because the follow-up time was short, postoperative functional analysis of traumatic hip arthritis was not included in this study.
Discussion
Computer technology is rarely applied in daily orthopedic traumatic surgery, but could make surgery easier and more precise; however, these computer systems require the use of new and expensive instruments in the operating room, and surgeons need to be familiar with their use.[Citation15,Citation16] In addition, it is very difficult to design a randomized controlled clinical trial to prove the benefit of computer-aided surgery.[Citation17] Even surgeons using these systems only master the basic functions, and are reluctant to explore all the advantages of these techniques.[Citation18] Furthermore, clinicians focus their efforts on surgical skills, and are unwilling to learn new and different technologies.
The use of VIRTOPS has been relatively rare in clinical orthopedics. Gellrich et al. [Citation19] set an uninjured eye orbit as a control, and successfully used VIRTOPS to reconstruct an injured eye orbit. Brown et al. [Citation20] also used VIRTOPS to design transcolumn screws in acetabular surgery. VIRTOPS could also be applied in complex artificial joint surgeries; VIRTOPS-based preoperative planning could help determine the positions of prostheses in difficult and complex acetabular revision surgery, as well as the reconstruction of complex acetabular functions.[Citation21,Citation22] In pelvic-acetabular surgeries, VIRTOPS could be used in minimally invasive techniques to calculate the trajectory and size of the screws, and can also be used for transcolumn screws.[Citation20,Citation23,Citation24]
When we used Mimics software to simulate a severely injured pelvis model, the result was more comprehensive and simplified the operation by simulating each step of the procedure. Because this required some knowledge of computer in addition to medical knowledge, the segmentation process of our model was carried out by the surgeons who had studied this software system. In this process, each model was observed, and compared with the 2D CT fault layer-by-layer, in order to reduce the errors automatically generated by a 3D model. This achieved greater accuracy than in models built by engineers who lacked medical knowledge.[Citation25] Thus, each step of the virtual surgery was controlled and presented by the doctors themselves.
Our experience showed that VIRTOPS could help us to understand complex acetabular fractures. Letournel [Citation26] emphasized that a surgeon should not undertake the treatment of acetabular fractures until they are fully understood. Despite modern diagnostic techniques including 3D and CT scanning, we found that understanding acetabular fractures was a gradual process, requiring deep analysis of each bone fragment, as well as the spatial relationships among bone fragments, by removing fragments or hyalinizing the fracture model. Despite careful preoperative planning, certain intraoperative ‘variations’ might still occur, and each step of VIRTOPS could minimize the effect of these variations.
In complex acetabular fractures, simulating the reduction and fixation of bone fragments from different angles can aid in the choice of the right surgical approach, as is needed in some transverse, ‘T’ type, or double-column fractures. Furthermore, simulation can sketch out the shape of the plate and the directions and precise lengths of the screws in the three sections of the multiplanar reconstruction. This even enabled preoperative selection and shaping of the internal implant, with postoperative CT scanning to re-evaluate the surgical results.
Among the 13 patients, the fixation methods in 12 cases were completely adopted after preoperative planning, and only one case had some minor changes. Changes in VIRTOPS are caused by different conditions in the virtual and actual surgeries. In a virtual environment, the bone fragments would not be attached to soft tissues so that the screws could be easily placed in any direction. However, in a real operation, the soft tissues would interfere with reduction, thus hindering the view of the operative field. In addition, because high-energy pelvic and acetabular fractures are often concurrent, there may be a delay until conditions became stable enough for surgery on an acetabular fracture; the reduction then becomes more difficult, and cannot be demonstrated by virtual surgery. Therefore, surgeons need to be aware of the actual conditions of the field of view in VIRTOPS.
The basic treatment principles in displaced acetabular fractures include anatomic reconstruction of the articular surface to restore its biomechanical characteristics, and stable fixation to allow immediate postoperative exercise. Acetabular surgery involves important and specific problems, including the choice of correct operative approach, reduction of bone fragments, and determination of fixation patterns. It is known that meticulous preoperative planning can ensure successful acetabular surgery. In this study, a computer-assisted simulation method based on CT data for reduction and fixation of acetabular fractures was developed for preoperative planning. This simulation method enabled performance of a complete virtual operation for an acetabular fracture on a 3D virtual pelvis acquired from real patient CT data. The planned operative approach and fixation patterns were followed in all cases. The results indicate that virtual reduction and fixation of fractures using different views can provide surgeons with a better understanding of fracture characteristics and aid in determination of the correct operative approach and fixation patterns. Furthermore, this virtual procedure has educational potential. Virtual operations can help teach younger and less experienced surgeons.
It is important for a surgeon to keep in mind the difference between virtual and real conditions. In a virtual environment, the fragments are not attached to soft tissue and the screws can easily be placed in all possible directions. However, the soft tissue interferes with the reduction and narrows the working space in the real operative field. Even when length and direction of the screws are accurately measured, they cannot technically be assessed in reality. That is why length and direction of the screws were not assessed in this study. However, we still believe that the determination of length and direction of the screws can help surgeons avoid screw perforation of the hip joint. Another drawback of this virtual surgical procedure is the requirement of relatively more time because the manual segmentation process is very time-consuming. The time needed for virtual planning increased with the severity of the fracture.
One limitation of this study is that it did not address the question of whether or not the use of virtual surgical planning results in better treatment outcomes. Ongoing studies should seek to assess the influence of virtual surgical planning on treatment outcomes in a prospective fashion. Another limitation is the small number of cases and a limited sample size of fracture types, which may limit the ability to generalize our results to large patient populations and more complex fracture types. The experience with applications for virtual surgery in fracture management has been limited because computer technologies are less commonly used in routine orthopedic practice. Moreover, surgeons focus more on technical skills than the use of technologies that require new and different knowledge. Greater usability and more widespread adoption of the method presented in this article depend on advances in software technologies and surgeons’ familiarity with their use. In this study, a virtual surgical procedure on a 3D model based on real CT data of acetabular fractures using commercially available computer software and personal computers was developed. The results demonstrated the high clinical relevance of virtual preoperative planning. In our opinion, this virtual procedure may be a valuable tool for surgeons to learn about the nature of an acetabular fracture and to formulate an appropriate surgical plan because it can simulate all the steps of a real procedure. Computer technologies will be increasingly introduced to help surgeons plan and perform operative procedures with greater precision. Our study may serve as an impetus for exploring the use of computer-assisted virtual surgical planning in clinical applications for orthopedic trauma.
Conclusion
A virtual surgical procedure for acetabular fractures is feasible and useful clinically in surgical planning. It may be a valuable tool for surgeons in learning about the nature of a fracture and in formulating an appropriate surgical plan. Our study may serve as an impetus for exploring the use of computer-assisted virtual surgical planning in clinical applications for orthopedic trauma.
Disclosure statement
All authors have no conflict of interest regarding this paper.
Funding
This study was supported by Basic Research Project of Science Technology and Innovation Committee of Shenzhen Municipality in 2016 (No. JCYJ20160427193559599) and Basic Research Project of Science and Technology Program of Baoan District in Shenzhen in 2016 (No. 2016CX166).
References
- Yu AW, Duncan JM, Daurka JS, et al. A feasibility study into the use of three-dimensional printer modelling in acetabular fracture surgery. Adv Orthop. 2015;2015:617046.
- Grubor P, Krupic F, Biscevic M, et al. Controversies in treatment of acetabular fracture. Med Arch. 2015;69:16–20.
- Tile M. Pelvic fractures: operative versus nonoperative treatment. Orthop Clin North Am. 1980;11:423–464.
- Deo SD, Tavares SP, Pandey RK, et al. Operative management of acetabular fractures in Oxford. Injury. 2001;32:581–586.
- Kumar A, Shah NA, Kershaw SA, et al. Operative management of acetabular fractures. A review of 73 fractures. Injury. 2005;36:605–612.
- Hu Y, Li H, Qiao G, et al. Computer-assisted virtual surgical procedure for acetabular fractures based on real CT data. Injury. 2011;42:1121–1124.
- Chen Y, Zhang K, Qiang M, et al. Computer-assisted preoperative planning for proximal humeral fractures by minimally invasive plate osteosynthesis. Chin Med J (Engl). 2014;127:3278–3285.
- Wu XB, Wang JQ, Zhao CP, et al. Printed three-dimensional anatomic templates for virtual preoperative planning before reconstruction of old pelvic injuries: initial results. Chin Med J (Engl). 2015;128:477–482.
- Kernan BT, Wimsatt JA. III, Use of a stereolithography model for accurate, preoperative adaptation of a reconstruction plate. J Oral Maxillofac Surg. 2000;58:349–351.
- Marchetti C, Bianchi A, Bassi M, et al. Mathematical modeling and numerical simulation in maxillo-facial virtual surgery (VISU). J Craniofac Surg. 2006;17:661–667.
- Zinser MJ, Mischkowski RA, Dreiseidler T, et al. Computer-assisted orthognathic surgery: waferless maxillary positioning, versatility, and accuracy of an image-guided visualisation display. Br J Oral Maxillofac Surg. 2013;51:827–833.
- Polley JW, Figueroa AA. Orthognathic positioning system: intraoperative system to transfer virtual surgical plan to operating field during orthognathic surgery. J Oral Maxillofac Surg. 2013;71:911–920.
- Li Y, Jiang Y, Zhang N, et al. Clinical feasibility and efficacy of using virtual surgical planning in bimaxillary orthognathic surgery without intermediate splint. J Craniofac Surg. 2015;26:501–505.
- Uribe F, Janakiraman N, Shafer D, et al. Three-dimensional cone-beam computed tomography-based virtual treatment planning and fabrication of a surgical splint for asymmetric patients: surgery first approach. Am J Orthod Dentofacial Orthop. 2013;144:748–758.
- Wong JM, Bewsher S, Yew J, et al. Fluoroscopically assisted computer navigation enables accurate percutaneous screw placement for pelvic and acetabular fracture fixation. Injury. 2015;46:1064–1068.
- Yang Y, Wang F, Han S, et al. Isocentric C-arm three-dimensional navigation versus conventional C-arm assisted C1-C2 transarticular screw fixation for atlantoaxial instability. Arch Orthop Trauma Surg. 2015;135:1083–1092.
- Mohsen AM, Phillips R. Letter of the reviewers – update on CAOS projects. Injury. 2004;35:S-A2–5.
- Langlotz F, Bächler R, Berlemann U, et al. Computer assistance for pelvic osteotomies. Clin Orthop Relat Res. 1998;354:92–102.
- Gellrich NC, Schramm A, Hammer B, et al. Computer-assisted secondary reconstruction of unilateral posttraumatic orbital deformity. Plast Reconstr Surg. 2002;110:1417–1429.
- Brown GA, Firoozbakhsh K, Gehlert RJ. Three-dimensional CT modeling versus traditional radiology techniques in treatment of acetabular fractures. Iowa Orthop J. 2001;21:20–24.
- Munjal S, Leopold SS, Kornreich D, et al. CT-generated 3-dimensional models for complex acetabular reconstruction. J Arthroplasty. 2000;15:644–653.
- Rommens PM, Hessmann MH. [Acetabulum fractures]. Unfallchirurg. 1999;102:591–610.
- Attias N, Lindsey RW, Starr AJ, et al. The use of a virtual three-dimensional model to evaluate the intraosseous space available for percutaneous screw fixation of acetabular fractures. J Bone Joint Surg Br. 2005;87:1520–1523.
- Tonetti J, Cloppet O, Clerc M, et al. Optimal placement of iliosacral screws: 3D computed tomography simulation. Rev Chir Orthop Reparatrice Appar Mot. 2000;86:360–369.
- Noser H, Radetzki F, Stock K, et al. A method for computing general sacroiliac screw corridors based on CT scans of the pelvis. J Digit Imaging. 2011;24:665–671.
- Letournel E. Acetabulum fractures: classification and management. Clin Orthop Relat Res. 1980;151:81–106.