Abstract
Objective: To examine the reliability of ultrasonographic thickness and compressibility along with real-time pressure monitoring to evaluate postmastectomy lymphedema and to suggest a reference range of appropriate pressure.
Design: Measurement reliability study.
Setting: Research laboratory.
Participants: Fifteen patients with prior mastectomy for breast cancer who were diagnosed with secondary lymphedema, and 16 healthy control subjects.
Methods: The thickness and compressibility of the subcutaneous layer in the arms of 15 postmastectomy patients with secondary lymphedema were measured using B-mode and M-mode ultrasonography. An ultrasound machine was equipped with a real-time pressure-monitoring device to monitor downward compression pressure on the arms at a constant velocity. The ratio of thickness change defined the compressibility index. Two different experienced examiners participated in the measurement of lymphedema. Intrarater reliability and inter-rater reliability were estimated using the intraclass correlation coefficient. Very good reliability was defined as an ICC of more than 0.8.
Main outcome measurements: The thickness of the subcutaneous layer, the compressibility index, and the intrarater and inter-rater reliability were measured.
Results: The measured thicknesses demonstrated very good intrarater and inter-rater reliability for the forearm and upper arm. For the compressibility index, the upper arm and forearm had very good intrarater and inter-rater reliability at over 2000 Pa of compression (>0.9).
Conclusions: Ultrasonography with real-time pressure monitoring may be useful for evaluating the severity and characteristics of lymphedema, particularly at compression pressures more than 2000 Pa.
1. Introduction
Lymphedema is a chronic progressive disease that causes swelling in subcutaneous tissues due to obstruction of lymphatic vessels or lymph nodes, resulting in accumulation of lymph fluid in the affected region.[Citation1] Lymphedema is one of the most common complications after mastectomy in patients with breast cancer.[Citation2,Citation3] Early diagnosis and treatment of lymphedema is indispensable for favorable clinical outcomes in patients with breast cancer.[Citation4] Reliable and accurate measurement of lymphedema is essential to assess disease severity at the time of diagnosis and treatment efficacy.[Citation4,Citation5]
Evaluation of lymphedema has most commonly been focused on the size and volume of the affected arm. The circumference of the affected arm is measured using a measuring tape and the volume is calculated by volumetry using water displacement or three-dimensional optoelectronic estimation.[Citation6,Citation7] However, these measurements cannot evaluate the physical properties of lymphedema tissue such as structural change, fibrosis, and hardness, which are known to affect the quality of life in patients with breast cancer.[Citation8,Citation9] Magnetic resonance imaging (MRI) and computed tomography (CT) have the advantage of evaluating physical characteristics and volume changes; however, MRI is expensive and CT exerts harmful effects from radiation.[Citation10] In contrast, the advantage of ultrasonography is that it can be performed at outpatient clinics and can evaluate physical characteristics and volume changes of lymphedema tissue in real time. Therefore, there have been several studies to evaluate the use of ultrasonography in lymphedema patients.[Citation11] Further, reports have confirmed changes in subcutaneous tissue through evaluation of the elasticity of these tissues, using ultrasound imaging.[Citation12,Citation13]
Previous studies revealed that ultrasonography had good intra- and inter-rater reliability in evaluating the subcutaneous tissue thickness in healthy subjects.[Citation14] Another study revealed that the compressibility of the affected arm in postmastectomy lymphedema patients decreased as compared to the contralateral arm and that the measurement of compressibility using ultrasonography with the maximal compression technique was a valuable tool for evaluating lymphedema.[Citation15] However, this method had a few limitations: the pressure was imprecisely controlled for and there were measurement problems in the evaluation of the subcutaneous tissue due to unequal maximal compression power performed by each examiner.
In this study, the thickness and compressibility of subcutaneous tissues in lymphedema were evaluated by ultrasonography with real-time pressure monitoring, and then, the intrarater and inter-rater reliability of this compression method were examined. In addition, our study determined which reference ranges of pressure points have the best reliability with minimal compressive influence on the lymphedema tissue in postmastectomy lymphedema patients.
2. Materials and methods
2.1. Subjects
2.1.1. Healthy subjects
Sixteen healthy volunteers with no history of edema, surgery or systemic disease were recruited and evaluated before the evaluation of lymphedema patients in order to confirm the effectiveness and safety of the real-time pressure monitoring system.
2.1.2. Secondary lymphedema patients after mastectomy
Patients who complained of upper extremity lymphedema were recruited at the outpatient clinic at Seoul National University Hospital in June 2011. All patients were diagnosed with secondary lymphedema by physicians who had more than 10 years of clinical experience. Among them, patients who consented to participate in this study were recruited. Inclusion criteria were completion of unilateral mastectomy for breast cancer and no current evidence of cancer. Patients, who had open skin lesions, had bilateral mastectomies or any signs of infection, such as cellulitis or lymphangitis, were excluded. In total, 15 patients were recruited from those who visited the outpatient clinic. Tumor stage, treatment method, height, weight and involved lymph nodes were reviewed through electronic medical records. This study was approved by the Institutional Review Board of Seoul National University Hospital (No. H-1103-104-356). The purpose and the procedures of this study were explained to the subjects and written informed consent was obtained from all of them, and the study was performed according to the IRB regulations and the principles of the Declaration of Helsinki.
2.2. Measurements
A high-back chair, a table, an ultrasound machine (Accuvix V20, A88891451003318, Medison Co., Seoul, Korea), and a real-time pressure monitoring device were prepared. The height of the chair and table were adjusted according to each subject's height. The height of the arms in sitting position was adjusted using 0.5, 1, and 2-cm acrylic plates. Subjects were asked to sit in the neutral position and place their arms on the table by flexing their arms forward with their forearm supinated.
All patients were evaluated by two physicians who had more than ten years of clinical experience with lymphedema. Examiners performed ultrasonography using a 7.5 MHz 38-mm linear-array transducer (L5-13IS, Medison Co., Seoul, Korea) equipped with a pressure sensor. The ultrasonographic measurements were performed on the upper arm between a point 10 cm proximal to the midpoint of the medial and lateral epicondyles of the humerus and the bicipital groove of the shoulder (). In addition, ultrasonographic measurement were performed on the forearm between a point 10 cm distal to the midpoint of the medial and lateral epicondyles and the midpoint of the radial and ulnar styloid processes (), as described in prior studies.[Citation14,Citation15] For simultaneous usage with ultrasonographic measurement, real-time pressure monitoring was obtained by a device that was specially designed for the precise measurement of thickness and pressure. This device could synchronize the ultrasonographic video clip and pressure monitoring with a specific mark on the real-time pressure monitoring program. For the simultaneous start of the ultrasonographic video clip and pressure monitoring program, each device was operated by foot switches lying side by side. The ultrasonographer stepped on these switches with 1 foot and started the ultrasonographic video clip and pressure monitoring program simultaneously (). The upper arm and forearm were compressed down at a constant velocity of 0.8 mm/sec by the transducer with the precision instrument and the images were saved with real-time. The pressure was checked by the manometer that was connected to the pressure monitoring program on the computer. To measure the real-time thickness of the subcutaneous tissue, the video clip of ultrasonographic M-mode was obtained. Sweep speed was 60 Hz and penetration was 2.5 cm. Examiners monitored the increasing pressure and the compressed subcutaneous tissue until its thickness no longer changed with additional pressures (). Minimal ultrasonographic gel was used to minimize its effect on pressure.
Figure 1. Measurement sites were centers of upper-arm and forearm. Three points were marked before measurement (A) the midpoint of the medial and lateral epicondyles, (B) the midpoint of the bicipital groove, (C) the midpoint of the radial and ulnar styloid processes). Two points were measured with ultrasonography 10 cm proximal and distal from A. These points were on the lines between A and B and between A and C.
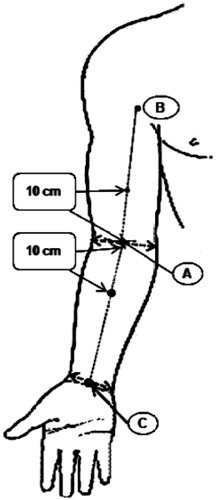
Figure 2. Ultrasonography (USG) with a linear transducer equipped with a pressure sensor was used to evaluate lymphedema tissue. Using a constant velocity controller, the arm was compressed down at a constant velocity using a precision instrument (0.8 mm/sec). The pressure signal was recorded in the computer using a data acquisition system.
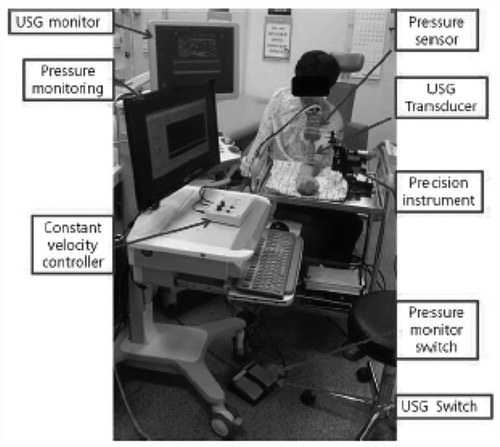
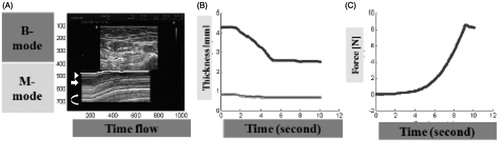
Figure 3. (A) Ultrasonographic B-mode and M-mode images were matched with real-time pressure measurements using Matlab. After confirmation of skin, subcutaneous tissue and muscle layer in B mode, M mode was started with pressure monitoring system. From the top of M mode, arrowhead is skin, arrow is subcutaneous tissue and the lighter gray space (curved arrow) is the muscle layer. Pressure increased constantly until the thickness of subcutaneous layer no longer changed. (B and C) It plotted the real-time change in thickness and force over time. Red and green lines are the thickness of subcutaneous tissue and skin with time. The blue line is the change of compressive force with time.
Two experienced examiners conducted the following procedures independently. Each examiner assessed the unaffected arm as a reference for comparison and then assessed the affected arm. Changes in the thickness of the subcutaneous tissue with increasing compressive forces were evaluated. In addition, the changing ratio of thickness was calculated using the following formula:
This ratio was defined as compressibility, which represents the degree of tissue deformation from its original configuration by compression.[Citation15]
One examiner re-assessed the affected arm for intrarater reliability, while the other examiner re-assessed the affected arm for inter-rater reliability. The mean time interval between individual assessments was 5 minutes, which was sufficient to allow the tissue thickness to recover.
Pressures and ultrasonographic images were synchronized in real-time by the Matlab 2007b (The Mathwork Inc., Natick, MA). To simultaneously obtain pressures and images, a foot switch for starting pressure monitoring was used when ultrasonographic images began to be captured. The thickness of the subcutaneous tissue was measured by marking the boundary between the dermis and muscle marked in green color. The thickness and the changing ratio of thickness according to the force applied were plotted by matching pressures and images ().
2.3. Statistical analysis
All statistical analyses were performed using SPSS 18.0 package (SPSS Inc., Chicago, IL). Demographic characteristics were compared between patients with right or left arm lymphedema. Categorical data were analyzed by Fisher’s exact test (two by two) and linear data by linear association (two by k). Continuous data were analyzed by the Mann–Whitney U-test. Statistical significance was defined as a p value of <.05. The thickness and the ratio of thickness change were plotted at every 100 Pa in healthy patients and every 500 Pa in lymphedema patients. The affected and unaffected arms were compared by Wilcoxon signed rank test. The intraclass correlation coefficient (ICC) was calculated for estimating intrarater reliability and inter-rater reliability. Very good reliability was defined as an ICC of more than 0.8.[Citation16] Inter- and intrarater reliabilities of the upper arm and forearm were plotted using Excel 2007 (Microsoft Corp., Redmond, WA).
3. Results
3.1. Demographic characteristics
The mean age of the healthy subjects was 32.8 ± 6.5 years (range: 27–48 years). They were all right handed. Measurements were performed on the right side in eight subjects and on the left side in eight subjects. Nine subjects were male and seven subjects were female. They had no history of edema, surgery, or systemic diseases that could affect the upper extremity’s volume.
The mean age of the lymphedema patients was 59.1 ± 8.8 years (range: 42–78 years). They were all right handed. Operations were performed on the right side in seven patients and on the left side in eight patients. There was no significant difference in height, weight, BMI, duration of lymphedema, the number of involved lymph nodes or tumor stage between the patients with right- or left-sided lymphedema. Treatment modality, including surgery, radiation, and chemotherapy, were not significantly different between the two groups. One patient underwent surgery and radiation alone, while all other patients underwent surgery, radiation, and chemotherapy. Tumor stages in participants were IIa in one patient, IIb in four patients, IIIa in four patients, and IIIb in five patients. The duration of lymphedema was 33.1 ± 26.8 months and the number of lymph nodes was 6.7 ± 6.4 ().
Table 1. Demographic characteristics of patients with lymphedema who underwent ultrasonographic measurements.
3.2. The thickness and the ratio of thickness change of subcutaneous tissue in healthy patients
The thickness of subcutaneous tissue decreased with increasing pressure; however, the ratio of thickness increased with increasing pressure. The thickness of the forearm and upper arm did not change when the pressure was more than 2000 Pa. ‘Maximal compression’ was previously defined as compression with a sufficient pressure such that additional pressure could not produce a noticeable decrease in the thickness of the soft tissue.[Citation14,Citation15] Based on this definition, the maximal compression in this experiment was obtained at 2000 Pa pressure. Furthermore, when the pressure was below 1000 Pa, the thickness did not change because the pressure was too small to produce change in the tissue thickness in healthy subjects. Therefore, the thickness and ratio were obtained for every 100 Pa from 1000 Pa to 2000 Pa. The thickness and the ratio of thickness with maximal compression of the upper arm were 1.16 ± 0.54 mm and 0.39 ± 0.11, respectively. For the forearm, they were 1.18 ± 0.54 mm and 0.37 ± 0.10 ().
Table 2. The thickness and the ratio of thickness change in upper arm and forearm by compression in healthy subjects.
3.3. Difference in thickness and the ratio of thickness change between affected and unaffected arms in lymphedema patients
The thickness of the affected side in both the forearm and upper arm had a tendency to be larger than that of the unaffected side. The differences in thickness between the affected and unaffected arms were statistically significant in terms of the pressure on the forearm, but not the upper arm. The thickness of the forearm and the upper arm were unchanged between 0 Pa and 500 Pa and when the pressure was more than 6000 Pa, which represents a wider range of pressures compared to healthy patients. The ratio of thickness change of the affected side in the forearm tended to be smaller than that of the unaffected side. There were no significant differences between the ratio of thickness of affected side and that of the unaffected side in the upper arm. For the ratio of thickness change on the forearm, p values were 0.05–0.08 for pressures between 1500 and 2500 Pa and pressures over 5500 Pa. However, the ratios at the other pressure ranges were significantly different. The thickness and the ratio of thickness change changed rapidly from 0 Pa to 2000 Pa in both affected and unaffected arms, but changed more slowly at pressures more than 2000 Pa. The thickness and the ratio of thickness with maximal compression of the upper arm were 2.60 ± 0.95 mm and 0.41 ± 0.05, respectively. In the forearm, they were 3.48 ± 1.58 mm and 0.31 ± 0.10, respectively ().
Table 3. Comparison of the thickness and the ratio of thickness change between unaffected and affected sides in upper arm and forearm by compression in lymphedema patientsTable Footnotea.
3.3.1. Intra- and inter-rater reliabilities for subcutaneous tissue thickness and the ratio of thickness change in healthy subjects
The overall intra- and inter-rater reliabilities of subcutaneous tissue thickness and the ratio of thickness in both the upper arm and forearm were very good, and the ICC was over 0.9 across the entire range of pressures ().
Table 4. Reliabilities of the thickness and the ratio of thickness in upper arm and forearm by compression in healthy subjectsTable Footnotea.
3.3.2. Intra- and inter-rater reliabilities for subcutaneous tissue thickness and the ratio of thickness change in lymphedema patients
The overall intra- and inter-rater reliability of measuring subcutaneous tissue thickness in both the upper arm and forearm had ICC scores greater than 0.8 over the entire range of pressures. For inter-rater reliability of ratio of thickness change in the upper arm, ICC exceeded 0.8 after compressing to 1000 Pa and more. Beyond 2000 Pa, ICC was maintained near 0.9 with minor fluctuation. Similarly, for inter-rater reliability in the forearm, ICC was below 0.8 from 1000 Pa to 1500 Pa. After compressing to 2000 Pa and more, ICC became more than 0.9. For intrarater reliability in both the upper arm and forearm, ICC was more than 0.8 across all pressure points. In addition, after compressing more than 2000 Pa, ICC exceeded 0.9 ().
Table 5. Reliabilities of the thickness and the ratio of thickness in upper arm and forearm by compression in lymphedema patientsTable Footnotea.
4. Discussion
Lymphedema is recognized as a secondary pathologic condition after breast cancer surgery.[Citation17,Citation18] In particular, lymphedema influences the morbidity of cancer survivors because it is a chronic, progressive and incurable disease.[Citation19,Citation20] The incidence of lymphedema has been reported to be 6–70%.[Citation21–24] The reason for this large disparity is that there is no consensus regarding the diagnosis and measurement of lymphedema.[Citation25] In general, many clinicians regard a difference in arm circumference between both upper limbs of 2 cm or more as a diagnostic criterion. However, because the sensitivity of this criterion is only 37%, the actual incidence may be much higher.[Citation26] Therefore, reliable, easy-to-use, noninvasive, and cost-effective measurement methods are necessary. In addition, if the measurement method could provide information on the texture and shape of the affected arms, it would be more useful in clinical practice.[Citation25,Citation27]
Although water displacement was previously regarded as the gold standard of volume measurement, it has some limitations in clinical application. For example, it could not be applied to patients with open skin lesions or localized edema. Also, it is impractical because disinfection of the equipment and water replacement are required after each measurement.[Citation28] The reliability of tonometry is no better than that of circumference measurement or volumetry. These results may be due to operational inconsistencies between assessments and raters, especially because it is not easy to apply stable force without fluctuation. In addition, it is difficult to ensure that a tonometer is held vertically against the skin.[Citation29] A perometer, an optoelectronic device using infrared light, is thought of as a reliable tool for measuring lymphedema because it is much faster, more hygienic and more accurate than volumetry in calculating volume change. Though the perometer is sensitive to volume change, it has some limitations in clinical application because it cannot measure characteristics of tissue such as fibrosis.[Citation30] Therefore, ultrasonography was selected for measuring and evaluating characteristics of the lymphedema tissue.
In a prior study, the measurement of the ratio of thickness change with maximal compression using ultrasonography was introduced as a useful tool for the evaluation of hardness of the skin and subcutaneous tissue.[Citation15] However, it is impossible for the operators to compress these tissues with equal force every time, which could produce unreliable results. Therefore, new methods are needed for more reliable and precise measurement of subcutaneous tissues in lymphedema patients. It was thought that ultrasonography using a real-time pressure control device would compress subcutaneous tissue with equal force in all subjects and improve the reliability of measurements in lymphedema.
In this study, ultrasonography with real-time pressure monitoring for the precise control of pressure was used and found to be a reliable tool for evaluating lymphedema. These measurements allowed for a more precise evaluation of the ratio of thickness change of the subcutaneous tissue with appropriate inter-rater and intrarater reliability. Before its application in lymphedema patients, healthy volunteers who had no past medical history were recruited to confirm the effectiveness and safety of the real-time pressure monitoring system. The thickness and the ratio of thickness change of subcutaneous tissues were measured and analyzed, followed by the intrarater and inter-rater reliabilities. Almost all the intraclass correlation coefficient (ICC) values were very good (ICC >0.9). These reliabilities results were better than the prior study utilizing the maximal compression technique but without the precise pressure control device (skin, 0.33 < ICC <0.73; subcutaneous tissue, 0.66 < ICC <0.83).[Citation14] These better results produced in this experiment are probably due to the precise control of pressure. In the previous study, there might have been some measurement problems in the evaluation of subcutaneous tissues because the thickness of subcutaneous tissue changed with changes in pressure, while the thickness of the muscle layer below the subcutaneous tissue also changed when the operators applied maximal compression. In contrast, our study measured the changes of the skin and subcutaneous tissue while minimizing any changes to the muscle layer below by precise pressure control, which probably led to more accurate measurements of tissue thickness and the ratio of thickness change. The inter-rater reliabilities in this study were also improved compared with the previous study.
After confirmation of effectiveness and safety of real-time pressure monitoring method in healthy subjects, this method was applied to lymphedema patients. The results between healthy subjects and lymphedema patients differed slightly. The thickness of unaffected subcutaneous tissues in lymphedema patients was larger than that of healthy subjects. This may be due to having nine young males in the healthy patient cohort, while all the lymphedema patients were women who generally had more fat in their subcutaneous tissue. In lymphedema patients, the thickness of the affected side in both the forearm and upper arm tended to be larger than that of unaffected side. The thickness of the forearm and upper arm did not change between 0 Pa and 500 Pa, which is lower than the 1000 Pa cutoff seen in healthy patients. It is known that the skin thickness of lymphedema patients tends to be thicker and the skin elasticity tends to be less than in healthy subjects.[Citation15,Citation19,Citation25] These characteristics of skin tissue in lymphedema patients might influence the initial pressure that could change the thickness; hence, the lower cutoff seen in our study. The thickness of subcutaneous tissue of lymphedema patients was greater than that of healthy subjects and the compressibility were less than that of healthy subjects. The thickness also stopped changing when the pressure was more than 6000 Pa in lymphedema patients, which is at a higher cutoff than that of healthy subjects.
The ratio of thickness change of the affected side in the forearm tended to be smaller than the unaffected side in lymphedema patients. There were no significant difference between the ratio of thickness change of the affected side and that of unaffected side in the upper arm. Lymphedema was not prominent in the upper arm and the thickness and ratio were not significantly different despite increasing pressures. For the ratio of thickness change on the forearm, p values were 0.05–0.08 for pressure between 1500 and 2500 Pa and over 5500 Pa. The values at all other pressures were significantly different. The thickness and the ratio of thickness change changed rapidly from 0 Pa to 2000 Pa in both affected and unaffected arms but changed more slowly at pressures more than 2000 Pa. After compression to more than 2000 Pa, the overall ICC was higher than 0.8 for both intra- and inter-rater reliability. This implies that examiners can accurately evaluate lymphedema with this compression method if they control pressure in a constant manner. In our study, the operators can measure the compressibility of subcutaneous tissue with proper and minimal pressure that can minimize the damage of lymphedema tissue. Based on this, a compression force of approximately 2000 Pa is recommended to evaluate the compressibility reliably and to minimize the damage to the lymphedema tissue. However, our sample size was small. Further studies are needed to verify our findings, validate this method, to determine whether this technique is reliable with a larger sample size, and determine how external factors, including elasticity and BMI, influence ultrasonographic measurements.
This is the first study to evaluate lymphedema using ultrasonography with real-time pressure monitoring. We obtained very good reliability at more than 2000 Pa of compression. This method had several advantages compared to other methods. We can also confirm the structure and characteristics of the subcutaneous tissue and the real-time pressure on the tissue more easily. If medical engineers develop a smaller, lighter, easy-to-use pressure probe, this method becomes an even more useful tool to determine the severity and characteristics of lymphedema in clinical practice.
5. Conclusion
In conclusion, the results of this study suggest that ultrasonography with real-time pressure monitoring may be useful for evaluating the severity and characteristics of lymphedema with a very good reliability at pressures more than 2000 Pa.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
This study is supported by a grant (04-2011-0150) from the Seoul National University Hospital Research Fund.
References
- Lymphology ISo. The diagnosis and treatment of peripheral lymphedema. 2009 Concensus Document of the International Society of Lymphology. Lymphology. 2009;42:51–60.
- Oremus M, Dayes I, Walker K, Raina P. Systematic review: conservative treatments for secondary lymphedema. BMC Cancer. 2012;12:6.
- Rockson SG, Rivera KK. Estimating the population burden of lymphedema. Ann N Y Acad Sci. 2008;1131:147–154.
- Stout Gergich NL, Pfalzer LA, McGarvey C, et al. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–2819.
- Schonholz SM. Preoperative assessment enables the early detection and successful treatment of lymphedema. Cancer. 2009;115:909. author reply 909–910.
- Sander AP, Hajer NM, Hemenway K, Miller AC. Upper-extremity volume measurements in women with lymphedema: a comparison of measurements obtained via water displacement with geometrically determined volume. Phys Ther. 2002;82:1201–1212.
- Taylor R, Jayasinghe UW, Koelmeyer L, et al. Reliability and validity of arm volume measurements for assessment of lymphedema. Phys Ther. 2006;86:205–214.
- Hormes JM, Bryan C, Lytle LA, et al. Impact of lymphedema and arm symptoms on quality of life in breast cancer survivors. Lymphology. 2010;43:1–13.
- Ridner SH, Dietrich MS, Kidd N. Breast cancer treatment-related lymphedema self-care: education, practices, symptoms, and quality of life. Support Care Cancer. 2011;19:631–637.
- Fumiere E, Leduc O, Fourcade S, et al. MR imaging, proton MR spectroscopy, ultrasonographic, histologic findings in patients with chronic lymphedema. Lymphology. 2007;40:157–162.
- Tassenoy A, Vermeiren K, van der Veen P, et al. Demonstration of tissue alterations by ultrasonography, magnetic resonance imaging and spectroscopy, and histology in breast cancer patients without lymphedema after axillary node dissection. Lymphology. 2006;39:118–126.
- Ashikaga T, Burns D, O'Brien P, et al. Texture analysis of post breast cancer lymphedema ultrasound images obtained using a portable device – a pilot study. Lymphat Res Biol. 2005;3:147–155.
- van der Veen P, Vermeiren K, Von Kemp K, et al. A key to understanding postoperative lymphoedema: a study on the evolution and consistency of oedema of the arm using ultrasound imaging. Breast. 2001;10:225–230.
- Kim W, Chung SG, Kim TW, Seo KS. Measurement of soft tissue compliance with pressure using ultrasonography. Lymphology. 2008;41:167–177.
- Lim CY, Seo HG, Kim K, et al. Measurement of lymphedema using ultrasonography with the compression method. Lymphology. 2011;44:72–81.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310.
- Bar Ad V, Cheville A, Solin LJ, et al. Time course of mild arm lymphedema after breast conservation treatment for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2010;76:85–90.
- Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92:1368–1377.
- Hayes SC, Janda M, Cornish B, et al. Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. J Clin Oncol. 2008;26:3536–3542.
- Roiland RA, Heidrich SM. Symptom clusters and quality of life in older adult breast cancer survivors. Oncol Nurs Forum. 2011;38:672–680.
- Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3:208–217.
- Ronka RH, Pamilo MS, von Smitten KA, Leidenius MH. Breast lymphedema after breast conserving treatment. Acta Oncol. 2004;43:551–557.
- Suami H, Chang DW. Overview of surgical treatments for breast cancer-related lymphedema. Plast Reconstr Surg. 2010;126:1853–1863.
- Vignes S, Arrault M, Dupuy A. Factors associated with increased breast cancer-related lymphedema volume. Acta Oncol. 2007;46:1138–1142.
- Armer JM. The problem of post-breast cancer lymphedema: impact and measurement issues. Cancer Invest. 2005;23:76–83.
- Bland KL, Perczyk R, Du W, et al. Can a practicing surgeon detect early lymphedema reliably? Am J Surg. 2003;186:509–513.
- Gerber LH. A review of measures of lymphedema. Cancer. 1998;83:2803–2804.
- Tierney S, Aslam M, Rennie K, Grace P. Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg. 1996;12:412–417.
- Chen YW, Tsai HJ, Hung HC, Tsauo JY. Reliability study of measurements for lymphedema in breast cancer patients. Am J Phys Med Rehabil. 2008;87:33–38.
- Lee MJ, Boland RA, Czerniec S, Kilbreath SL. Reliability and concurrent validity of the perometer for measuring hand volume in women with and without lymphedema. Lymphat Res Biol. 2011;9:13–18.
