Abstract
Purpose: In oncological orthopedics, navigation systems are limited to use in specialized centers, because specific, expensive, software is necessary. To resolve this problem, we present a technique using general spine navigation software to resect tumors located in different segments.
Materials and Methods: This technique requires a primary surgery during which screws are inserted in the segment where the bone tumor is; next, a CT scan of the entire segment is used as a guide in a second surgery where a resection is performed under navigation control. We applied this technique in four selected cases. To evaluate the procedure, we considered resolution obtained, quality of the margin and its control.
Results: In all cases, 1 mm resolution was obtained; navigation allowed perfect control of the osteotomies, reaching the minimum wide margin when desired. No complications were reported and all patients were free of disease at follow-up (average 25.5 months).
Conclusions: This technique allows any bone segment to be recognized by the navigation system thanks to the introduction of screws as landmarks. The minimum number of screws required is four, but the higher the number of screws, the greater the accuracy and resolution. In our experience, five landmarks, placed distant from one another, is a good compromise. Possible disadvantages include the necessity to perform two surgeries and the need of a major surgical exposure; nevertheless, in our opinion, the advantages of better margin control justify the application of this technique in centers where an intraoperative CT scanner, synchronized with a navigation system or a dedicated software for bone tumor removal were not available.
Introduction
Computer-assisted navigation techniques are frequently used in spine surgery to obtain perfect control of screw insertion and in hip and knee replacement surgery where these techniques allow plane evaluation for osteotomies, restoring greater physiologic axis.[Citation1–4]
The first applications of computer-assisted surgery (CAS) systems in oncological orthopedics have had promising results; unfortunately, dissemination is limited to specialized centers due to the necessity of specific software.
In this paper, we present our technique in which we use the general spine navigation software to perform resections of bone tumors located in the limbs. Preliminary results from the first four patients are presented for indications, advantages, disadvantages, pitfalls and tricks that may be helpful for surgeons.
Materials and methods
The navigation system was a Brainlab ‘kolibri 2.0’; the software used for navigation was ‘Spine & Trauma 3D’; the dedicated osteotomes were produced by Brainlab and represent a three-dimensional landmark system recognized by the navigator.
Technique description
An adequate preoperative study is required to verify whether this technique is suitable or not (; case 4 ). The patient was studied based on the diagnosis. In case of a malignant tumor, staging was done. In all cases, an MRI was performed to better identify the tumor’s extension. A preliminary approach was performed, visualizing the bone immediately next to the tumor, paying careful attention not to contaminate the future wide margin (). Under C-Arm system control, five screws (length: 8 mm – diameter: 3.5 mm) were inserted in the bone (). The access was closed and the patient underwent a high resolution CT scan, including the tumor area, and the bone segment where the screws were inserted.
Figure 1. Preoperative MRI and CT scan showing a low-grade chondrosarcoma of the proximal humerus. Images demonstrate that a wide minimal resection sparing the joint is technically possible.
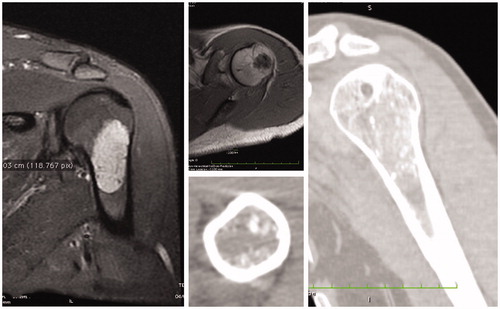
Figure 2. (A) The first incision (a) is performed on the same line as the incision necessary for tumor removal (b), as close as possible to the tumor, with a wide margin; (B) an intraoperative view of the five screws inserted in the bone as fiducial landmarks; (C) the scout-view showing the landmark screws.
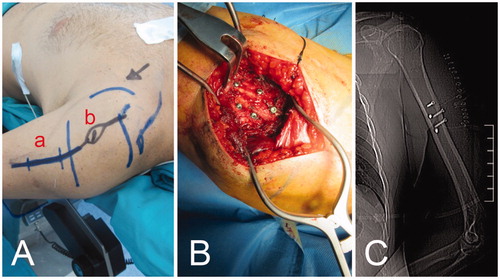
Table 1. Patients characteristics and surgeries.
In the second case, the procedures were then simulated through a dedicated dock-station where a fusion with the previously performed MRI was done, because the borders of the tumor were not clearly identifiable at CT scan.
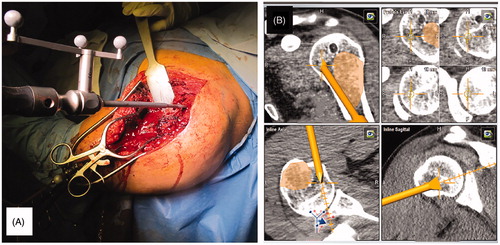
Within the following days, the patient underwent a second operation. The new incision was designed to remove the biopsy track and prolong the previous incision (), reopening the previous access and visualizing the screws; a landmark system was inserted into the normal bone, as close to the tumor as possible (). Before using the CAS system, the infrared navigation camera was positioned in order to see the landmarks and surgery area. The navigation probe was used to direct the camera and the computer to the right positions of the previously inserted screws, using the ‘Paired Point Matching’ registration method (). The CAS system identified the screws to recognize the patient’s position.
Figure 3. (A) The second incision is designed to remove the biopsy track; (B) a picture highlighting the first approach, opened a second time and the landmark system inserted into normal bone; (C) the navigation probe is used to show the screws’ position to the camera following the ‘paired point matching’ technique; (D) the dedicated osteotomies are calibrated and positioned for camera recognition.
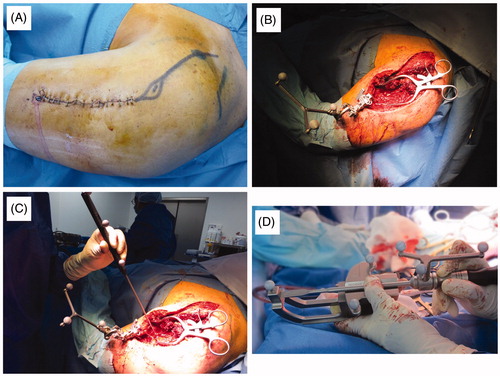
The obtained accuracy was revealed by the software and confirmed by the surgeon verifying the correspondence between a real point touched with the exploring probe and the corresponding one on the CT-3D reconstruction on the navigation system’s screen.
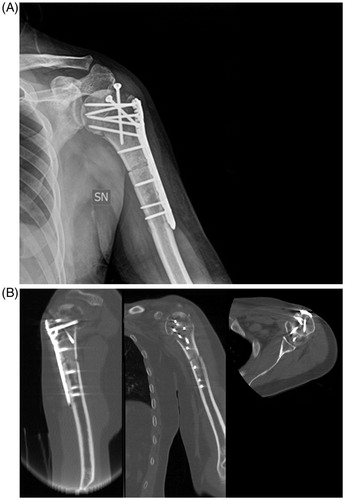
Successively, dedicated osteotomes were recognized by the navigation camera () and used for resection (), continuously controlling the margin on the navigation screen (). The reconstruction was performed in a traditional way, which depends on what the case necessitates. The approach was then sutured. The postoperative X-ray and CT-scan displayed a satisfying reconstruction ().
Alternative technique (case 3)
The first approach was performed directly under CT guidance; five K-wires were inserted under local or general anesthesia, in normal bone, near the affected bone. The high definition CT scan was performed and used for navigation and resection. The obtained accuracy and the histological margin were considered as the primary endpoints; secondary endpoints were the onset of complications and local recurrence.
The specimens were analyzed by a dedicated pathologist trained in muscular-skeletal tumors; all excision margins were painted with India or China ink. Successively, 1 cm sections of the specimen were performed and the distance between the inked surface and the tumor was measured. Supplementary sections were performed based on the macroscopic examination or the surgeon’s suggestion; the goal of the surgeries was to resect the bone within 1 cm from the tumor, as near as possible without an intralesional violation.
Infections, hematomas and iatrogenic fractures were considered complications. The patients were followed up every 3 months for the first 2 years with an MRI or a CT scan to detect local recurrence.
The procedures were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. All Patients were informed of the advantages and disadvantages of this technique. They gave consent for surgery and case publication.
Results
Landmark screws positioning was performed in every case, without incidents, even if in the first case, one of the five screws was dislocated. In three out of four cases, five screws were inserted as landmarks in the normal bone; in the fourth, four k-wires were inserted under local anesthesia and sedation.
After using the CAS system, the software estimated an accuracy of 1 mm, judged adequate to perform resection; the resolution was verified in every case by the surgeon. Macroscopic examination of the surgical specimen demonstrated wide margin; the relative sections and histologies confirmed a good control of the resection margin which was wide and inferior to 1 cm and of 2–3 mm when needed. No complications were reported and all patients were free of disease at follow-up (average, 25.5 months).
Discussion
Computer-assisted navigation is very effective to aid orthopedic surgery for trauma, joint replacement, cruciate ligament reconstruction and spine surgery;[Citation1–5] and is routine for several brain approaches in neurosurgery.[Citation6] More recently, computer-assisted navigation was applied in bone tumor resections where a reduction of the number of accidental intralesional margin surgeries was demonstrated.[Citation7,Citation8]
Computer-assisted navigation surgery was firstly performed in sacral and periacetabular tumors where the complex anatomy grants an easier recognition of landmarks.[Citation9–11] Since it is more difficult in iliac wings and limbs, some authors proposed using K-wires positioned as guide under CT-control or custom-made resection masks which allow to reproduce the intended osteotomy on the real patient.[Citation12–14] The availability of specific software for bone tumor resection could consent a safer approach, nevertheless they are only present in referred centers.
The present technique may allow surgeons to use the navigation dock already present in most hospitals for bone tumor resections too. The normal software allows recognition of specific points in the bone segment. This process is very simple on the spine, where the posterior arc is tri-dimensionally complex and it is easy to match the points required by the software with the corresponding points on the patient, as indicated by the pointer (Region Matching). This process is impossible on limbs or iliac wings. The proposed technique allows the introduction of landmarks, which are easily recognized by the navigator.
In 2004, Hufner et al published three cases of sacral tumor resections performed under computer-assisted navigation control. They used pins inserted in the iliac wing in local anesthesia as fiducial landmarks and a spine software to recognize them.
Cho et al. in 2009,[Citation15] published a similar technique in which the landmarks were K-wires, inserted in the operative theater under fluoroscopy control, but in their cases the objective was just to perform the osteotomies, sparing the joint; in our cases the navigated osteotomes allowed us to perfectly contour the tumor.
Even if in one case we used K-wires, for independent problems, we prefer screws because their recognition is probably more reliable and the second surgery can even be done after the first one. Ieguchi et al.,[Citation16] in 2012, published a series of 16 patients who underwent biopsies and excisions of soft tissue and bone tumors, in which the landmarks were 10 skin markers. They reported an average accuracy of 0.93 mm with a mean difference between the planned margin and postoperative CT or excised histologic specimen variable from 0 to 4 mm.
The minimum number or screws the software requires for the paired matching is four, but the greater the number of screws, the higher the accuracy and resolution. In our experience, five landmarks is a good compromise between the need of good resolution and the reduction of contamination.
Indeed, in the first case we performed, one of the screws was not sufficiently fixed to the bone and in the second surgery, during the calibration, it proved to be mobilized, but the presence of a previously inserted fifth screw allowed us to complete the calibration procedure and then the resection, so five landmarks guarantee that navigation may proceed, even when one landmark is not reliable.
Obviously, a greater number of screws and a higher distance between them should assure a better resolution but a larger oncological contamination; indeed, in case of local recurrence the area accessed for the screws’ insertions has to be removed en-bloc with the mass.
The screws should be arranged three-dimensionally and should be distant from one another as possible to ensure a firm insertion; the screws should be as small as possible to reduce variability when they are pointed for pair matching. Indeed, the screws we used were the thinnest screws routinely available in our hospital.
The screws during the first surgery and the landmark system during the second surgery should be inserted in an area close to the tumor because the accuracy of the navigation decreases if the distance between them grows.
When performed correctly, this technique allows perfect control of the margin, as shown in ; possible disadvantages include the necessity to perform two surgeries and the need of a major surgical exposure and a relative oncological contamination; nevertheless, in our opinion, the possible advantages of better margin control justify the application of this technique in centers where an intraoperative CT scanner synchronized with navigation is not available.
Figure 6. The anatomical specimen of a low-grade chondrosarcoma of the proximal humerus, showing the small wide margin achieved.
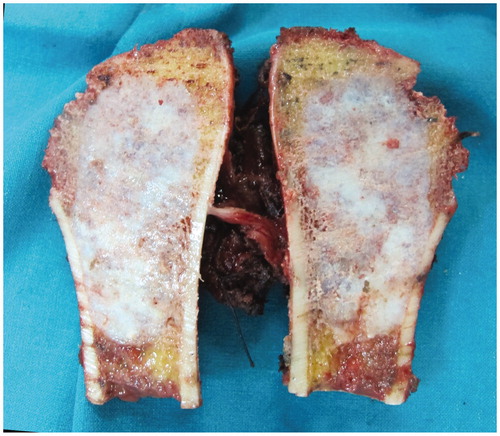
This technique is particularly suitable for cases where a small wide margin is advisable, such as for low-grade bone tumors, like low-grade chondrosarcoma (cases 3 and 4), and when an extremely precise osteotomy is advocated, as for acetabular osteotomy in extra-articular hip joint resection (case 1), or when the bone stock must be spared in condylar resection (cases 2 and 3).
Compliance with ethical standards
The procedures were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. All Patients were informed of the advantages and disadvantages of this technique. They gave consent for surgery and case publication.
Disclosure statement
The authors declare that they have no conflict of interest.
No funding was received for the present paper.
References
- Fu Y, Wang M, Liu Y, Fu Q. Alignment outcomes in navigated total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20:1075–1082.
- Rahmathulla G, Nottmeier EW, Pirris SM, et al. Intraoperative image-guided spinal navigation: technical pitfalls and their avoidance. Neurosurg Focus. 2014;36:E3. doi: 10.3171/2014.1.FOCUS13516.
- Fujishiro T, Nakaya Y, Fukumoto S, et al. Accuracy of pedicle screw placement with robotic guidance system: a cadaveric study. Spine (Phila Pa 1976). 2015;40:1882–1889.
- Liu Z, Gao Y, Cai L. Imageless navigation versus traditional method in total hip arthroplasty: a meta-analysis. Int J Surg. 2015; 21:122–127.
- Goradia VK. Computer-assisted and robotic surgery in orthopedics: where we are in 2014. Sports Med Arthrosc. 2014;22:202–205.
- Barone DG, Lawrie TA, Hart MG. Image guided surgery for the resection of brain tumours. Cochrane Database Syst Rev. 2014;CD009685. doi: 10.1002/14651858.CD009685.
- Jeys L, Matharu GS, Nandra RS, Grimer RJ. Can computer navigation-assisted surgery reduce the risk of an intralesional margin and reduce the rate of local recurrence in patients with a tumour of the pelvis or sacrum? Bone Joint J. 2013;95B:1417–1424.
- Wong KC, Kumta SM. Use of computer navigation in orthopedic oncology. Curr Surg Rep. 2014;2:47. doi: 10.1007/s40137-014-0047-0.
- Cho HS, Kang HG, Kim HS, Han I. Computer-assisted sacral tumor resection. A case report. J Bone Joint Surg Am. 2008;90:1561–1566.
- Hufner T, Kfuri M, Jr, Galanski M, et al. New indications for computer-assisted surgery: tumor resection in the pelvis. Clin Orthop Relat Res. 2004;426:219–225.
- Krettek C, Geerling J, Bastian L, et al. Computer aided tumor resection in the pelvis. Injury. 2004;35:S-A79–83.
- Zoccali C, Rossi B, Ferraresi V, et al. A multiplanar complex resection of a low-grade chondrosarcoma of the distal femur guided by K-wires previously inserted under CT-guide: a case report. BMC Surg. 2014;14:52. doi: 10.1186/1471-2482-14-52.
- Blakeney WG, Day R, Cusick L, Smith RL. Custom osteotomy guides for resection of a pelvic chondrosarcoma. Acta Orthop. 2014;85:438–441.
- Holzapfel BM, Pilge H, Prodinger PM, et al. Customised osteotomy guides and endoprosthetic reconstruction for periacetabular tumours. Int Orthop. 2014;38:1435–1442.
- Cho HS, Oh JH, Han I, Kim HS. Joint-preserving limb salvage surgery under navigation guidance. J Surg Oncol. 2009;100:227–232.
- Ieguchi M, Hoshi M, Takada J, et al. Navigation-assisted surgery for bone and soft tissue tumors with bony extension. Clin Orthop Relat Res. 2012;470:275–283.