Abstract
Purpose: Tumor-induced osteomalacia (TIO) is a rare paraneoplastic syndrome usually caused by phosphaturic mesenchymal tumors. Segmental resection has been recommended for these tumors in the bones because curettage was found to be associated with a high local recurrence rate. Navigation-assisted surgery provides radiological information to guide the surgeon during surgery. No previous study has reported on the efficacy of navigation-assisted surgery for tumors in patients with TIO. Therefore, the present study aimed to evaluate the efficacy of navigation-assisted surgery for tumors in patients with TIO.
Methods: The study included seven patients with TIO who were treated between January 2003 and December 2014 at our hospital. All patients underwent surgical treatment with or without the use of a 3-dimensional (3D) fluoroscopy-based navigation system. The laboratory data and oncological outcomes were evaluated.
Results: The follow-up period was 8–128 months. The tumors were located at the femur (n = 4), ischium, spine and ilium (n = 1). Of the seven patients, five underwent navigation-assisted surgery and two underwent surgery without navigation assistance. In the two patients who underwent surgery without navigation assistance, a complete cure was not obtained and osteomalacia did not resolve. One of these two patients and the other five patients who underwent navigation-assisted surgery, one patient had incomplete resection due to massive invasion of the tumor into the spinal canal, but five patients achieved complete excision and recovered from osteomalacia.
Conclusions: Navigation-assisted surgery using a 3D fluoroscopy-based navigation system is effective for tumors in patients with TIO.
Background
Tumor-induced osteomalacia (TIO) is a rare paraneoplastic syndrome usually associated with phosphaturic mesenchymal tumors (PMTs) [Citation1]. This condition is characterized by high levels of fibroblast growth factor-23 (FGF-23) secreted by the tumors, causing reduced reabsorption of phosphate in the proximal renal tubule, which results in hypophosphatemia and hyperphosphaturia [Citation2]. PMTs are usually benign and slow-growing masses. Detection of these tumors is challenging because they can present as ill-demarcated lesions anywhere in the body, including soft tissues and bones. The major clinical symptoms are non-specific, including bone pain, fractures and muscle weakness or fatigue, and the duration between symptom onset and diagnosis is very long [Citation3,Citation4].
Surgical treatment is recommended for patients with TIO in the bones, although there are several medical approaches [Citation5–7]. If the tumor is not completely excised, patients with TIO cannot be cured [Citation8,Citation9]. One-third of these tumors have been reported to occur in the bones, and the options for surgical treatment of tumors in the bones are tumor curettage and tumor segment resection [Citation7]. Previous studies have recommended segmental resection because tumor curettage was found to be associated with a high local recurrence rate [Citation8,Citation9].
Navigation-assisted surgery provides radiological information to guide the surgeon during surgery, and it has been reported to help safely resect malignant bone tumors, resulting in improved oncological and functional outcomes [Citation10,Citation11].
To our knowledge, no previous study has reported the efficacy of navigation-assisted surgery for tumors in patients with TIO. Therefore, the present study aimed to evaluate the efficacy of navigation-assisted surgery for tumors in patients with TIO.
Materials and methods
Patients
The present study included seven patients with TIO in the bones, who were treated between January 2003 and December 2014 at out hospital. This study was approved by our hospital’s institutional review board. The inclusion criteria were adult patients who were diagnosed with TIO in the bones, underwent at least one surgical treatment, and underwent at least 8 months of clinical follow-up. All patients underwent surgical treatment with or without the use of a 3-dimensional (3D) fluoroscopy-based navigation system. The study design was reviewed and approved by the ethics committee of our hospital.
Preoperative preparation
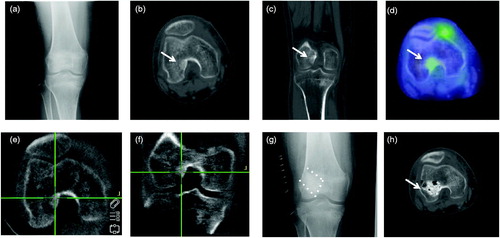
All patients suspected of having TIO were referred to our Orthopaedic Department from the Endocrinology Department. Multiple examinations were performed to identify the tumor, including venous sampling of FGF-23, positron emission tomography/computed tomography (CT) and magnetic resonance imaging ()) [Citation1,Citation8].
Figure 1. A 40-year-old female patient with the tumor of distal femur (#4). (a) Antero-posterior radiograph did not show the tumor clearly. (b, c) Axial and coronal views of computed tomography showed an obscure sclerotic lesion at the lateral epicondyle near the joint space. (d) Positron emission tomography showed positive FDG uptake in the tumor. (e, f) Axial and coronal views of the intraoperative 3D C-arm image. (g) Curettage was performed with the navigation system and reconstructed with artificial bone. (h) Postoperative computed tomography confirmed complete resection of the tumor.
Surgical procedures
Tumor curettage was chosen for tumors in the extremities, ischium and spine, and en bloc resection was chosen for tumors in the ilium. A 3D fluoroscopy-based navigation system was used as follows. A reference frame (Orthopaedic Frame HC; Medtronic, Louisville, CO) was attached rigidly to the bone near the tumor (femur, ilium or spinous process) with two half-pins or a bone clamp at the start of surgery. Intraoperative 3D images were then acquired with the C-arm of the Arcadis Orbic 3D device (Siemens AG, Erlangen, Germany). The C-arm of the image intensifier was equipped with a wireless tracker (Stealth Active wireless tracker S/N 130; Medtronic, Louisville, CO) for navigation registration. The acquired imaging data were transferred to a navigation computer (StealthStation TRIA plus; Medtronic, Louisville, CO), and a 3D image of the bone with the tumor was reconstructed and was displayed on the computer screen. The location of the curettage or resection site was confirmed with a passive planar blunt probe (SureTrak2 Universal Tracker, Large Passive Fighter; Medtronic, Louisville, CO) (). After curettage, the 3D C-arm was again used intraoperatively. If curettage was found to be incomplete, it was repeated. After final curettage, the tumor cavity was treated with high-temperature electrical coagulation and high speed burring. An artificial bone graft or bone cement was used to reconstruct any defects after curettage (). The surgical approach and number of procedures were recorded.
Postoperative monitoring and follow-up
Serum phosphorus levels were measured 24 hours, 48 hours, 72 hours, four days, five days and seven days after surgery, and every 3–4 days thereafter until normalization of the serum phosphorus level or discharge. FGF-23 levels were measured 24, 48 and 72 hours after surgery. All the patients were asked to visit the Orthopaedic and Endocrinology Department for follow-up at 2–3 months after surgery, and every 3–6 months thereafter. Endocrinology physicians administered medical therapies to patients whose serum phosphorous levels did not return to normal. Successful treatment was defined as pain relief and normal serum phosphorus and FGF-23 levels at the last follow-up.
Statistical analysis
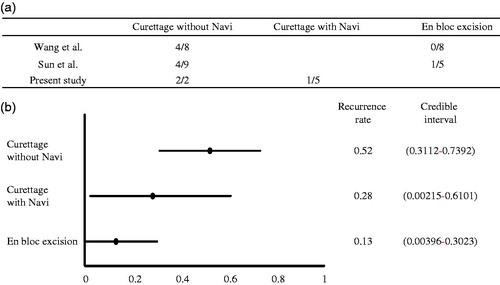
Bayesian estimation was performed to compare the recurrence rates among three different surgical methods: curettage without navigation, curettage with navigation, and en bloc excision. We used the surgical result of two previous case series [Citation8,Citation9] and that of the present study as prior probability distribution. In Bayesian statistics, parameter estimates are obtained as precision-weighted averages of the observed data likelihood function and prior distribution. The influence of prior distribution helps stabilize and anchor Bayesian estimates in the presence of small samples [Citation12]. Beta distribution was used for the prior distribution in this study. The recurrence rates were estimated using the Bayesian Markov chain Monte Carlo approach with binomial model. To identify the significant difference in recurrence rate, the highest posterior density (HPD) intervals were used. All data analyses were performed using SAS software (version 9.1.4; SAS Institute Inc., Cary, NC).
Results
Patient characteristics
The patient characteristics are presented in . The study included four male and three female patients. The mean age of the patients was 56 years (range, 40–64 years). The minimum follow-up period was 8 months (range, 8–128 months). The tumors were located at the femur (n = 4), ischium (n = 1), spine (n = 1) and ilium (n = 1), and the mean tumor size was 28.8 mm (range, 5–60 mm).
Table 1. Characteristics of the patients with tumor-induced osteomalacia.
Treatment and outcomes
The data on treatment and outcomes are presented in and . Of the seven patients, five underwent navigation-assisted surgery and two underwent surgery without navigation assistance. In the two patients (#3 and 7) who underwent surgery without navigation assistance, we could not distinguish the tumor from normal cancellous bone with the naked eye, and complete cure was not obtained and osteomalacia did not resolve. One of these two patients (#3) accepted secondary surgery with navigation assistance. In four patients who underwent primary navigation-assisted surgery and one patient who accepted secondary surgery with navigation assistance, complete curettage was achieved and osteomalacia was cured. In one patient with a spinal tumor who underwent primary navigation-assisted surgery, osteomalacia did not resolve because of incomplete resection due to massive invasion of the tumor into the spinal canal and vertebral body despite a two-stage anterior and posterior surgery. Intraoperative 3D C-arm imaging was performed to examine the existence of residual tumor, and additional curettage was performed in two patients (#3 and 5). The mean surgical time was 3.8 hours (range, 1.5–8.3 hours), and the mean blood loss was 674.3 mL (range, 0–2105 mL). The FGF-23 level normalized within 24 hours after the surgery in all successful cases. The serum phosphorus and alkaline phosphatase levels returned to normal in all successful cases at 3.6 days (range, 3–5 days) and 10.6 months (range, 6–18 months), respectively, after surgery (). The clinical symptoms of the patients improved over the follow-up period.
Table 2. Preoperative and postoperative laboratory values.
Comparison among three surgical methods
We compared three surgical methods: curettage without navigation, curettage with navigation, and en bloc excision. We excluded a case of en bloc resection with navigation system in our case to compare the efficacy of navigation system in the curettage of tumors. Bayesian estimation revealed that the recurrence rates (95% HPD intervals) of curettage without navigation, curettage with navigation, and en bloc resection were 0.52 (0.31–0.74), 0.28 (0.002–0.61) and 0.13 (0.004–0.3), respectively ().
Pathological findings
All patients had benign PMTs with limited mitosis. Immunohistochemical staining was positive for FGF-23 in all patients.
Discussion
Complete excision is the treatment of choice for most patients with TIO [Citation9,Citation10]. PMTs are usually small and ill-demarcated lesions that occur in the epiphysis; hence, the detection of these tumors is difficult [Citation1]. In this study, navigation-assisted curettage with high-speed burring and en bloc resection was completely accomplished in all patients, except one patient with a spinal tumor. The curative rates of osteomalacia with and without navigation-assisted surgery were 83% and 0%, respectively. Bayesian estimation revealed that practical use of the navigation system reduced the rate of recurrence to half of that when using curettage. Therefore, patients with TIO were successfully treated with navigation-assisted surgery using a 3D fluoroscopy-based navigation system.
Studies have recommended wide resection to achieve complete treatment of TIO because approximately 50% of patients experienced local recurrence because of insufficient curettage [Citation9,Citation10]. It is believed that the high local recurrence rate is associated with the difficulty to distinguish tumors from normal bones during surgery. In one patient (#2), we macroscopically divided the specimen into normal bone and tumor; however, microscopically, tumor cells were present in the expected normal bone (data not shown). Another reason for the high local recurrence rate is that tumor access is challenging because TIO might be localized almost at the epiphysis and might be eccentrically present, as was noted in some of our patients (#2, 3, 4 and 5) [Citation10].
The 3D C-arm enables visualization of the tumor location. Therefore, to overcome this issue and the above-mentioned problems, we used a navigation system. Navigation-assisted surgery for a benign bone tumor has been shown to be safe and useful for evaluating the location of deep benign bone tumors and confirming the position of tumors during curettage in real time [Citation13]. A previous study reported that navigation assistance helped to successfully prepare small holes for the removal of deeply located osteoid osteomas [Citation14]. Although the tumors were small and deep in some of our patients (#2, 4 and 5), the navigation system helped to successfully detect the tumors. Therefore, navigation-assisted surgery for TIO may be indicated in cases in which the tumor is located inside the medullary cavity of long bones or the pelvic bone, in which identification of the anatomical landmark is difficult. Furthermore, curettage with navigation system is recommended for preservation of the surrounding bone and joint in cases in which the tumor is benign or intermediate, and located in the bone. In contrast, in cases in which the cortical bone is extensively destructed by the tumor or malignant cases, en bloc excision is recommended.
Navigation systems can be categorized into image-based systems, such as CT and 2D and 3D fluoroscopy, and non-image-based systems. In the present study, we used a 3D fluoroscopy-based navigation system. This system can easily identify the location of the tumor in the bone and hence allows for minimal surgery. Furthermore, after excision, the 3D C-arm was again used intraoperatively to check whether the tumor was completely excised. Incomplete excision was identified in two patients in the post-excision scan, and further excision of the tumor was performed. This intraoperative examination to determine the adequacy of tumor excision can assist surgeons in treating TIO sufficiently and safely.
The present study has some limitations. The use of a 3D fluoroscopy-based navigation system requires the placement of a reference frame on the bone with the tumor, using two half-pins, which necessitates additional skin incisions and the drilling of holes in the bone. The 3D fluoroscopy-based navigation system has been used in anterior cruciate ligament reconstruction surgery, and no complications associated with pin insertion were reported [Citation15]. No adverse effects were observed in this study. Therefore, the 3D fluoroscopy-based navigation system can be used safely for treating bone tumors. Additionally, this study is limited by the small number of patients. Further studies with a larger number of patients and a longer follow-up duration are required to compare the efficacy of navigation-assisted surgery with that of surgery without navigation assistance in patients with TIO. However, a comparative study might be difficult to perform owing to the infrequency of TIO.
In summary, navigation-assisted surgery using a 3D fluoroscopy-based navigation system is effective for tumors in patients with TIO. We believe that navigation assistance with CT can help achieve complete curettage of tumors in patients with TIO and reduce the local recurrence rate.
Acknowledgements
Informed consent was obtained from all individual participants included in the study.
Disclosure statement
The authors report no conflicts of interest.
References
- Fukumoto S. Diagnostic modalities for FGF23-producing tumors in patients with tumor-induced osteomalacia. Endocrinol Metab (Seoul). 2014;29:136–143.
- Endo I, Fukumoto S, Ozono K, et al. Nationwide survey of fibroblast growth factor 23 (FGF23)-related hypophosphatemic diseases in Japan: prevalence, biochemical data and treatment. Endocr J. 2015;62:811–816.
- Carpenter TO. Oncogenic osteomalacia—a complex dance of factors. N Engl J Med. 2003;348:1705–1708.
- Ledford CK, Zelenski NA, Cardona DM, et al. The phosphaturic mesenchymal tumor: why is definitive diagnosis and curative surgery often delayed? Clin Orthop Relat Res. 2013;471:3618–3625.
- Hesse E, Rosenthal H, Bastian L. Radiofrequency ablation of a tumor causing oncogenic osteomalacia. N Engl J Med. 2007;357:422–424.
- Tutton S, Olson E, King D, et al. Successful treatment of tumor-induced osteomalacia with CT-guided percutaneous ethanol and cryoablation. J Clin Endocrinol Metab. 2012;97:3421–3425.
- Jiang Y, Xia WB, Xing XP, et al. Tumor-induced osteomalacia: an important cause of adult-onset hypophosphatemic osteomalacia in China: report of 39 cases and review of the literature. J Bone Miner Res. 2012;27:1967–1975.
- Wang H, Zhong D, Liu Y, et al. Surgical treatments of tumor-induced osteomalacia lesions in long bones: seventeen cases with more than one year of follow-up. J Bone Joint Surg Am. 2015;97:1084–1094.
- Sun ZJ, Jin J, Qiu GX, et al. Surgical treatment of tumor-induced osteomalacia: a retrospective review of 40 cases with extremity tumors. BMC Musculoskelet Disord. 2015;16:43.
- Cho HS, Oh JH, Han I, et al. The outcomes of navigation-assisted bone tumour surgery: minimum three-year follow-up. J Bone Joint Surg Br. 2012;94:1414–1420.
- Ieguchi M, Hoshi M, Takada J, et al. Navigation-assisted surgery for bone and soft tissue tumors with bony extension. Clin Orthop Relat Res. 2012;470:275–283.
- Song X, Lee S. Basic and advanced Bayesian structural equation modeling: with applications in the medical and behavioral sciences. Hoboken (NJ): Wiley; 2012. p. 36.
- Lee HI, Shim JS, Jin HJ, et al. Accuracy and limitations of computer-guided curettage of benign bone tumors. Comput Aided Surg. 2012;17:56–68.
- Raux S, Abelin-Genevois K, Canterino I, et al. Osteoid osteoma of the proximal femur: treatment by percutaneous bone resection and drilling (PBRD). A report of 44 cases. Orthop Traumatol Surg Res. 2014;100:641–645.
- Taketomi S, Inui H, Nakamura K, et al. Three-dimensional fluoroscopic navigation guidance for femoral tunnel creation in revision anterior cruciate ligament reconstruction. Arthrosc Tech. 2012;1:95–99.