Abstract
Technical difficulty of an operation is associated with patient and disease characteristics, indicating the necessity for surgeons to exercise patient-specific preparation. Such methods have been shown to be effective in the simulation suite, however, application in a real clinical environment has been sporadic. This systematic review attempts to answer if patient-specific preparation in challenging surgical procedures is feasible. A systematic review of OvidMedline, Embase and all Evidence Based Medicine review databases, was conducted in search of studies who described surgical rehearsals in all specialties. Following the application of defined inclusion and exclusion criteria relevant data were extracted and summarised. Descriptive synthesis was performed for all included studies and meta-analysis of data was applied when possible. Of fourty-nine studies included, thirty-seven were case-series, ten were non-randomised comparative trials and two randomised controlled trials. Accuracy of applied methods ranged from 66.7 to 100% and a good outcome was seen in 60–100% of operations. Meta-analysis of studies comparing rehearsals to real procedures (same patients) showed that simulated procedures were significantly faster than real ones (SMD = −1.56 [−2.19, −0.93] p < 0.00001) but were similar in other outcomes (fluoroscopy time: SMD = −0.1 [−0.63, 0.42] p = 0.7, fluoroscopy volume: SMD = −0.43[−0.97, 0.11], p = 0.12). Meta-analysis of studies comparing pre-operative rehearsals to standard treatment (two distinct groups of patients), demonstrated that real procedures were performed quicker if pre-operative rehearsal took place (SMD = −0.47 [−0.79, −0.16], P = 0.003) but the immediate clinical outcome was similar for practiced and not practiced operations (SMD =0.03[−0.23, 0.29], p = 0.82). Current evidence suggests that patient-specific pre-operative preparation is feasible and safe and decreases operational time.
Introduction
Patient and disease-specific characteristics are associated with an operation’s technical complexity [Citation1–6]. Hence, a methodical preparation towards challenging surgical procedures, encompassing patient-specific features, may have a significant effect on improving both the quality and outcome of surgery. Emerging technologies, such as 3D image reconstruction (e.g. fusion of 2D- into 3D-images using computed tomography (CT) slides), and rapid prototyping (building a physical model using computer aided design (CAD) software, e.g. 3D printing) facilitate the effective and accurate construction of patient-specific anatomical models, surgical guides and implants [Citation7–10], thus opening new avenues for pre-operative rehearsals.
Despite the increase in popularity within the last twenty years the feasibility, accuracy and clinical impact of patient-specific pre-operative preparations have not been well established [Citation7, Citation8, Citation11], impeding their uptake into routine clinical practice. The primary aim of this review was to evaluate whether patient specific pre-operative preparation is feasible in the time-pressured environment of surgical specialties. Moreover, safety, accuracy and clinical impact were assessed.
Methods
This systematic review was performed according to the PRISMA guidelines [Citation12]. A systematic review of OvidMedline (1946-July 31st 2015), Embase (1947-July 31st 2015) and all Evidence Based Medicine (EBM) review databases, including Cochrane databases, was conducted using the following terms: “patient”, “specific”, “simulat*”, “surg*”, “planning”, “patient specific” and “rehearsal”. The search strategy was validated with the identification of eight studies familiar to the authors and believed to be appropriate for inclusion. Review papers were searched by hand for additional studies appropriate for inclusion.
Inclusion criteria
Studies on patient-specific pre-operative rehearsals or planning followed by the performance of real-time operations were included in this review. The term “rehearsals” is used here to reflect physical re-enactment using virtual surgical tools (i.e. similar to the ones used in real surgery), whilst planning refers to viewing an anatomical model and deciding on a surgical strategy with or without the use of virtual tools that are dissimilar to the ones used in a real theatre. Only studies written in English were included.
Exclusion criteria
Studies reporting merely construction of anatomical models or simulators without subsequent real-time operations were excluded because the aim of the review was to assess clinical impact of the preparation process. Case reports, reviews, editorials, abstracts and conference proceedings were excluded from further analysis. Studies on peri-operative navigation without pre-operative preparation were also excluded. Also, studies exclusively on paediatric or foetal surgery were excluded.
Identification of relevant studies
Two authors independently assessed studies identified through the literature search for relevance, by reading the title and abstract. Full text was retrieved for all studies potentially meeting the eligibility criteria.
Data extraction
Data extraction from selected studies was done using a Microsoft® Excel spreadsheet, which included the following categories: authors, country, year of publication, anatomical model type, type of surgery, number of participants, rehearsal/planning process, number of cases pre-operative planning was followed in, clinical outcomes, other outcomes, cost and time requirements for preparation of pre-operative planning session. Data extraction was conducted independently by two authors and subsequently validated by a third. Descriptive synthesis was performed for all included studies, structured around the PICO (Participants, Intervention, Comparators, Outcome measure) approach [Citation13].
Meta-analysis
For the purposes of the meta-analysis assumption of normal distribution of the data was made and the following mathematical equations were used to calculate Standard Deviation (SD) on different occasions (collaboration):
SD = IQR (Interquartile range) width/1.35
SD = √N (CI (Confidence Interval) upper limit – CI lower limit)/4.128
SD = SE (Standard Error)/√(1/NE+1/Nc), SE = Mean Difference (MD)/t
N: sample size
NE: sample size of intervention group
Nc: sample size of control group
t: ratio of difference in means to standard error of difference in means
Results
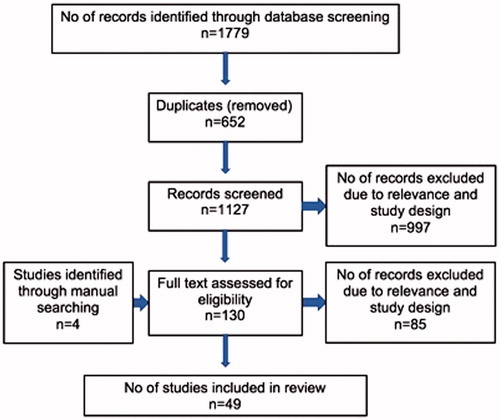
The literature search yielded 1779 records. Of these, 652 were duplicate studies and were therefore excluded. Titles and abstracts of the remaining 1127 studies were assessed for relevance, by two of the authors. Full text was acquired for 130 studies. From these, 45 matched the inclusion criteria. Manual search of references (from review studies identified during the literature search) yielded 4 additional studies for inclusion ().
Study characteristics and description
Studies were published between 1998 and 2015 (). Of fourty-nine studies matching the inclusion criteria, 37 were case-series, 10 were non-randomised comparative trials and 2 randomised controlled trials. The most common surgical specialities in the included studies were maxillofacial surgery (n = 16) [Citation7, Citation8, Citation14–27] and orthopaedics (n = 15) [Citation9, Citation10, Citation28–40] ().
Table 1. Characteristics of included studies
Participants
The participants were patients due to undergo surgery. Whilst most preparation processes involved only the surgeon, in 2 studies the entire surgical team was involved aiming to achieve improved coordination [Citation41, Citation42].
Type and content of intervention
The methods for patient-specific preoperative preparation included: i) surgical planning; allowing the surgeon to establish the surgical approach and dissection sequence, but not including a complete physical rehearsal of the procedure e.g. inspection of anatomy with augmented reality environment platforms [Citation37, Citation43], or ii) surgical rehearsal (i.e. simulated surgery), where the surgeon had a variety of virtual surgical tools at their disposal and performed all the physical and mental processes one would in a real theatre environment e.g. performing surgery on a virtual reality simulator [Citation41, Citation44–46].
For the purposes of surgical preparation, Patient-Specific (PS) three-dimensional virtual and synthetic anatomical models were built through the processes of 3D image reconstruction [Citation11, Citation29, Citation31, Citation37, Citation41, Citation43–48] and additive manufacturing [Citation23, Citation49] (i.e. Stereolithography [Citation7, Citation50]) respectively. In some studies, physical cutting guides and implants were designed and manufactured during the rehearsal process and then sterilised and used during the real operation [Citation11, Citation29, Citation31, Citation37, Citation41, Citation43–48].
A variety of medical imaging modalities are used for image 3D reconstruction including various types of Magnetic Resonance Imaging (MRI) [Citation11, Citation29, Citation31, Citation37, Citation41, Citation43–48], Computed Tomography (CT) [Citation7, Citation8, Citation10, Citation11, Citation23, Citation29, Citation31, Citation37, Citation41, Citation44–47, Citation49, Citation50] and more specialised imaging such as Diffusion Tensor Imaging (DTI) [Citation43].
Comparators
Two types of comparisons were used in the included studies: (i) results of rehearsals were compared to the ones of real operations (i.e. patients act as their own controls) [Citation41, Citation44, Citation45] (ii) preoperative preparation methods were compared to standard treatment or computed aided surgery (i.e. two distinct groups of patients) [Citation15, Citation21, Citation38, Citation51]. The overall outcome in question was similarity between rehearsal and real procedure on the first occasion and assessment of possible superiority of the rehearsed procedures compared to non-rehearsed ones (evaluating for possible differences) in the second type of comparisons.
Type of outcomes
Descriptive synthesis of data regarding measured outcomes yielded the following commonly emerging themes: (i) accuracy of pre-operative preparation methods and (ii) clinical outcomes.
Accuracy of pre-operative preparation methods
Accuracy of the pre-operative preparation methods is reported using several outcome measures (). The most frequently used were (i) number of cases in which the preoperatively formulated plan was successfully followed [Citation7, Citation10, Citation14–16, Citation19, Citation20, Citation25, Citation27–29, Citation31, Citation32, Citation34, Citation35, Citation37, Citation39, Citation47, Citation49, Citation52, Citation53] (ii) anatomical accuracy of models compared to operative anatomy [Citation7, Citation46], and/or (iii) validity of the pre-operative processes, as reported by surgeons/surgical team [Citation41, Citation44–46, Citation48]. The results for accuracy range from 66.7–100% ().
Figure 2. Above: Similarity between anatomy and surgical plan during rehearsal compared with real procedure. Size of circles is indicative of sample size. Below: Face and content validity of preoperative preparation processes.
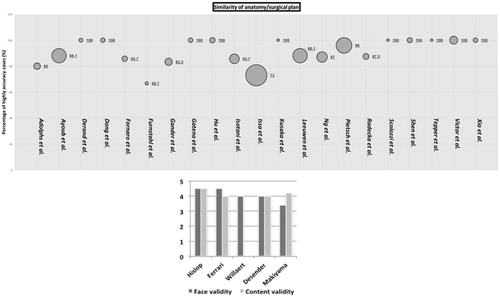
Table 2. Accuracy of pre-operative preparation methods and immediate surgical results. Additional information regarding accuracy and clinical results can be found in , and .
Clinical outcomes
Immediate surgical outcomes, including peri-operative complications, were reported in most studies. For comparison, the number of cases that had a satisfactory outcome (e.g. anatomical reduction of a fracture) are presented as a percentage of the overall number of cases ().
The majority of studies reported satisfactory immediate surgical results (60–100%). Complication reporting was sparse with two exceptions: (i) van Steenberghe et al. who reported 6/27 cases of bruxism (involuntary grinding of the teeth), a complication known to be associated with immediate loading of implants in fully endentulous maxillae [Citation50], and (ii) Fürnstahl et al. [Citation10] who describe severe pain postoperatively due to incomplete procedure [medial compartment of the knee not repaired in theatre, despite having achieved repair during rehearsal]. The number of participants was too small (n = 3) to embark on further analysis ().
Surgeons’ feedback
Face validity [the extent to which a simulation appears similar to the real situation] [Citation59] and content validity [validity of tests based on detailed examination of its contents] [Citation60] where reported as above 3.4/5, demonstrating good realism ().
Authors found the preparation methods to be useful in different ways such as: (i) better understanding of patient individual anatomy and pathology [Citation11, Citation15, Citation16, Citation19, Citation28, Citation29, Citation31, Citation39, Citation41, Citation43, Citation44, Citation46–49, Citation54, Citation55], (ii) reducing operating time or number of procedures required [Citation7–9, Citation16, Citation18, Citation20, Citation23, Citation24, Citation28, Citation37, Citation44, Citation49, Citation50], (iii) reducing complications or assisting in the avoidance of damage to vital structures [Citation7, Citation8, Citation23, Citation29, Citation31, Citation37, Citation41, Citation43–48], and (iv) promoting a minimally invasive approach (e.g. reduce amount of dissection needed) [Citation7, Citation29, Citation37, Citation43]. Drawbacks were also highlighted, for example technical issues encountered during the application of pre-operative rehearsals, including reliance of anatomical models on quality of imaging for accuracy [Citation9, Citation11, Citation23, Citation41, Citation45, Citation47, Citation49]. Definition can be increased by increasing the number of images or acquiring thinner slices [Citation9, Citation47, Citation49], however, additional resources would be needed and the ionisating radiation dose received by patients may increase [Citation7]. In fact, three of the included studies reported that additional imaging was required [Citation9, Citation10, Citation49].
Technical troubleshooting [Citation11, Citation23, Citation41, Citation43, Citation45] as well as unrealistic biomechanical properties, mostly concerning soft tissue, [Citation11, Citation31, Citation41, Citation43–47] have been reported. On occasion, the contralateral, healthy, side was used as a template for the repair of the diseased one [Citation15, Citation18, Citation20, Citation25, Citation26, Citation39, Citation40], which could be troublesome in cases of bilateral pathology or asymmetry.
It should also be noted that many authors propose PS preoperative preparation for “difficult” cases (e.g. severely comminuted fractures or cranial base tumours surrounded by vital structures) [Citation7, Citation9, Citation11, Citation23, Citation47, Citation48] and find no additional benefit in what they would traditionally consider a “straightforward” case [Citation7, Citation10, Citation23, Citation44, Citation46].
A number of studies reported the cost of pre-operative preparation with PS anatomical models, ranging from hundreds to thousands pounds [Citation14, Citation15, Citation25, Citation36, Citation54]. The manufacturing time ranged from hours to weeks [Citation16, Citation19, Citation28, Citation55].
Quality assessment of included studies
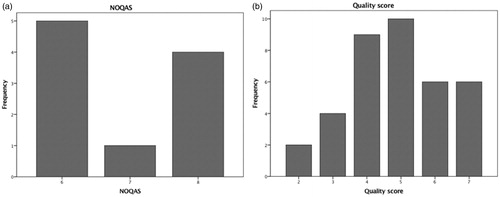
The quality of the studies was appraised using the Cochrane Collaboration’s Tool for Assessing Bia [Citation61], the Newcastle – Ottawa Quality Assessment Scale (NOQAS) [Citation62] and three minute case series appraisal tool [Citation63] for RCTs, cohort and case control studies and case series respectively ().
RCTs
Introduction of bias to the study conducted by Small et al. [Citation38] is very unlikely because enough information is given regarding the randomisation and the concealment allocation processes. The assessors of the outcome were blinded and there was sufficient completeness of outcome data. The authors reported both positive and negative results, reducing the possibility of reporting bias. There do not, however, appear to have taken into account personnel blinding, which would be practically very difficult [Citation38].
In comparison, Ayoub et al. [Citation15] do not provide information on how randomisation took place or if there was allocation concealment or personnel, and patient and assessor blinding. In regards to the reported outcomes there were data provided about the clinical results but not about the accuracy of the pre-operative preparation technique. Finally, introduction of reporting bias is unlikely because both positive and negative results are reported [Citation15].
Case-series and non-randomised trials
The majority of case series and non-randomised trials are well designed as shown in . All non-randomised trials scored six or more stars out of maximum nine and most case series had more than five (out of maximum eight) elements of well-designed case series.
Meta-analysis
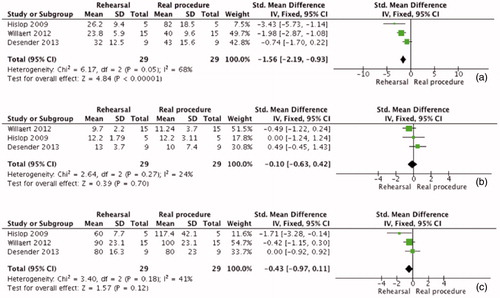
Meta-analysis was applied where permitted by similarity of the research question and the measured outcomes. Hislop et al. [Citation44], Desender et al. [Citation41] and [Citation45] all assessed the likeness of rehearsals and real procedures in vascular surgery and employed three common outcomes to do so (i.e. time to complete procedure, fluoroscopy time and fluoroscopy volume) (). The results of the meta-analysis demonstrate that although rehearsals were significantly quicker than real procedures (SMD = −1.56 [−2.19, −0.93] P < 0.00001), the other two outcomes measured during the rehearsal, resembled the results of the real procedure (fluoroscopy time (min): SMD = −0.1 [−0.63, 0.42] P = 0.7, fluoroscopy volume (ml): SMD= −0.43 [−0.97, 0.11] P = 0.12).
Figure 4. Meta-analysis comparing rehearsals and real procedures. a. Comparison of time to complete procedure b. Comparison of fluoroscopy time c. Comparison of fluoroscopy volume.
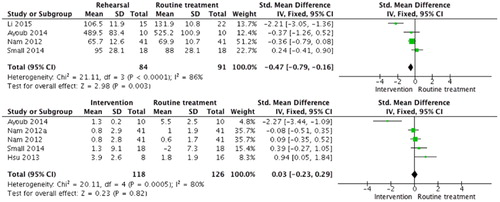
Li et al. [Citation51], Ayoub et al. [Citation15], Small et al. [Citation38], and Hsu et al. [Citation21] recruited two distinct groups of patients to compare the clinical results of surgical procedures that were rehearsed to standard surgical treatment, while Nam et al. [Citation9] compared the former to real time computer assisted surgery. The pre-rehearsed operations were completed in significantly less time (SMD −0.47 [−0.79, −0.16] P = 0.003) but the immediate clinical outcome was similar for practiced and not practiced operations (SMD =0.03 [−0.23, 0.29] P = 0.82) ().
Figure 5. Meta-analysis of rehearsal compared with standard or other intervention (e.g. Nam et al patient specific preparation compared to computer assisted surgery). a. Time (min) to complete procedure. b. Linear translation (mm) of rehearsal outcome compared to the one of real procedure. Two different measured outcomes are included for Nam et al (for femoral and tibial compartment).
Discussion
This review demonstrates that patient specific pre-procedural are feasible and safe. The accuracy of the anatomical models and the immediate clinical outcomes of the rehearsed procedures were satisfactory. Meta-analysis comparing rehearsals and real procedures showed good correlation, whilst when operations following rehearsals were compared to non-rehearsed ones the former were performed quicker and yielded the same surgical outcome. Practical issues in the design and manufacturing of anatomical models are also highlighted in this review and can act as focus points for future studies.
The potential of patient-specific pre-procedural rehearsals shown in our review is also highlighted in the current literature. For example, [Citation64] conducted a review on the use of 3D printing in medicine, giving a favourable opinion regarding the usefulness of additive manufacturing techniques for patient-specific preoperative planning and for building bespoken implants and prosthetics. However, the authors recognise that their conclusions are limited by the small number of cases currently reported and should be validated by larger scale trials.
Similarly, Pratt et al. [Citation65] looked into preparation prior to foetal surgery, suggesting that although early evidence is promising, the exact impact on surgical outcomes cannot be accurately determined due to small numbers of reported cases. A beneficial impact was also reported by See et al. [Citation66], investigating the impact of endovascular surgery rehearsals on trainees’ performance. The lack of randomised controlled trials was highlighted in both studies.
This systematic review has some limitations. Only two RCTs are included in this review whilst a significant volume of the presented evidence stems from case-series and non-randomised trials. This is a frequent occurrence in literature regarding emerging technologies in surgery [Citation67]. Whilst some studies have shown case series to have a wider range of measured outcomes and estimates of effect size when compared to RCTs [Citation68, Citation69], and to report the best achievable outcome rather then the one routinely accomplished [Citation70], others do not find these conclusions to be true. For instance, Linde et al. [Citation71] showed non-randomised trials to have consistent results to RCTs. Similarly, Dalzier et al. found a good correlation of case series and RCT results [Citation68]. Nevertheless, case-series can demonstrate feasibility and safety.
Application of meta-analysis was limited by the diverse outcome measures employed in various studies and was reflected in the high heterogeneity found mostly in the comparison of two distinct groups of patients (I2 test 80% an 86%). This could be due to various reasons, one of them being small sample size in each study, which is known to be associated with high heterogeneity [Citation72]. Further, the potential variance in population, surgical specialty and intervention implementation may confound the results [Citation73]. However, as we are inquisitive about the application of PS rehearsals in all surgical specialties we felt it was important to continue with this analysis and report the results.
Although the introduction of pre-operative rehearsals in routine clinical care should be preceded by the provision of high level evidence, they are worth exploring because they may lead to increased efficiency and reduced operating time, whilst maintaining safe outcomes. Rapid technological advances are expected to augment the accuracy of these procedures and reduced manufacturing times for virtual and synthetic anatomical models may increase the applicability of patient-specific preprocedural rehearsals.
Additional information
Funding
References
- Chopra S, Satkunasivam R, Kundavaram C, Liang G, Gill IS. Outlining the limits of partial nephrectomy. Transl Androl Urol. 2015;4(3):294–300.
- Hou W, Yan W, Ji Z. Anatomic features involved in technical complexity of partial nephrectomy. Urology. 2015;85(1):1–7.
- Kim JY, Kim YW, Kim NK, Hur H, Lee K, Min BS, et al. Pelvic anatomy as a factor in laparoscopic rectal surgery: a prospective study. Surg Laparosc Endosc Percutan Tech. 2011;21(5):334–9.
- Martel G, Al-Suhaibani Y, Moloo H, Haggar F, Friedlich M, Mamazza J, et al. Neoadjuvant therapy and anastomotic leak after tumor-specific mesorectal excision for rectal cancer. Dis Colon Rectum. 2008;51(8):1195–201.
- Polewczyk A, Kutarski A, Czekajska-Chehab E, Adamczyk P, Boczar K, Polewczyk M, et al. Complications of permanent cardiac pacing in patients with persistent left superior vena cava. Cardiol J. 2014;21(2):128–37.
- Vivek MA, Augustine AJ, Rao R. A comprehensive predictive scoring method for difficult laparoscopic cholecystectomy. J Minim Access Surg. 2014;10(2):62–7.
- Tepper OM, Sorice S, Hershman GN, Saadeh P, Levine JP, Hirsch D. Use of virtual 3-dimensional surgery in post-traumatic craniomaxillofacial reconstruction. Journal of Oral & Maxillofacial Surgery. 2011;69(3): 733–41.
- Haq J, Patel N, Weimer K, Matthews NS. Single stage treatment of ankylosis of the temporomandibular joint using patient-specific total joint replacement and virtual surgical planning. Br J Oral Maxillofac Surg. 2014;52(4):350–5.
- Nam D, Maher PA, Rebolledo BJ, Nawabi DH, McLawhorn AS, Pearle AD. Patient specific cutting guides versus an imageless, computer-assisted surgery system in total knee arthroplasty. Knee. 2013;20(4):263–7.
- Furnstahl P, Vlachopoulos L, Schweizer A, Fucentese SF, Koch PP. Complex Osteotomies of Tibial Plateau Malunions Using Computer-Assisted Planning and Patient-Specific Surgical Guides. J Orthop Trauma. 2015;29(8):e270–6.
- Kockro RA, Serra L, Tseng-Tsai Y, Chan C, Yih-Yian S, Gim-Guan C, et al. Planning and simulation of neurosurgery in a virtual reality environment. Neurosurgery. 2000;46(1):118–35; discussion 35-7.
- GRADE Working Group 2004 Sb, Guyatt 2008a, Guyatt 2008. The GRADE approach. Available from: http://handbook.cochrane.org/chapter_12/12_2_1_the_grade_appro ach.htm.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Adolphs N, Liu W, Keeve E, Hoffmeister B. RapidSplint: virtual splint generation for orthognathic surgery - results of a pilot series. Comput Aided Surg. 2014;19(1-3):20–8.
- Ayoub N, Ghassemi A, Rana M, Gerressen M, Riediger D, Holzle F, et al. Evaluation of computer-assisted mandibular reconstruction with vascularized iliac crest bone graft compared to conventional surgery: a randomized prospective clinical trial. Trials. 2014;15:114.
- Derand P, Rannar LE, Hirsch JM. Imaging, virtual planning, design, and production of patient-specific implants and clinical validation in craniomaxillofacial surgery. Craniomaxillofac Trauma Reconstr. 2012;5(3):137–44.
- Dhanda J, Cooper C, Ellis D, Speculand B. Technique of temporomandibular joint replacement using a patient-specific reconstruction system in the edentulous patient. British Journal of Oral & Maxillofacial Surgery. 2011;49(8):618–22.
- Essig H, Rana M, Kokemueller H, von See C, Ruecker M, Tavassol F, et al. Pre-operative planning for mandibular reconstruction - a full digital planning workflow resulting in a patient specific reconstruction. Head Neck Oncol. 2011;3:45.
- Gander T, Essig H, Metzler P, Lindhorst D, Dubois L, Rucker M, et al. Patient specific implants (PSI) in reconstruction of orbital floor and wall fractures. J Craniomaxillofac Surg. 2015;43(1):126–30.
- Gateno J, Xia JJ, Teichgraeber JF, Christensen AM, Lemoine JJ, Liebschner MA, et al. Clinical feasibility of computer-aided surgical simulation (CASS) in the treatment of complex cranio-maxillofacial deformities. J Oral Maxillofac Surg. 2007;65(4):728–34.
- Hsu SS, Gateno J, Bell RB, Hirsch DL, Markiewicz MR, Teichgraeber JF, et al. Accuracy of a computer-aided surgical simulation protocol for orthognathic surgery: a prospective multicenter study. J Oral Maxillofac Surg. 2013;71(1):128–42.
- Lo LJ, Chen YR, Tseng CS, Lee MY. Computer-aided reconstruction of traumatic fronto-orbital osseous defects: aesthetic considerations. Chang Gung Medical Journal. 2004;27(4):283–91.
- Perry M, Banks P, Richards R, Friedman EP, Shaw P. The use of computer-generated three-dimensional models in orbital reconstruction. British Journal of Oral & Maxillofacial Surgery. 1998;36(4):275–84.
- Schepers RH, Raghoebar GM, Vissink A, Stenekes MW, Kraeima J, Roodenburg JL, et al. Accuracy of fibula reconstruction using patient-specific CAD/CAM reconstruction plates and dental implants: A new modality for functional reconstruction of mandibular defects. J Craniomaxillofac Surg. 2015;43(5):649–57.
- Scolozzi P. Maxillofacial reconstruction using polyetheretherketone patient-specific implants by "mirroring" computational planning. Aesthetic Plastic Surgery. 2012;36(3):660–5.
- Steinbacher DM. Three-Dimensional Analysis and Surgical Planning in Craniomaxillofacial Surgery. J Oral Maxillofac Surg. 2015;73(12 Suppl):S40–56.
- Xia JJ, Gateno J, Teichgraeber JF, Christensen AM, Lasky RE, Lemoine JJ, et al. Accuracy of the computer-aided surgical simulation (CASS) system in the treatment of patients with complex craniomaxillofacial deformity: A pilot study. J Oral Maxillofac Surg. 2007;65(2):248–54.
- Dong Y, Dong Y, Hu G, Xu Q. Three-dimensional reconstruction of extremity tumor regions by CT and MRI image data fusion for subject-specific preoperative assessment and planning. Comput Aided Surg. 2011;16(5):220–33.
- Fornaro J, Keel M, Harders M, Marincek B, Szekely G, Frauenfelder T. An interactive surgical planning tool for acetabular fractures: initial results. J Orthop Surg Res. 2010;5:50.
- Franceschi JP, Sbihi A, Computer Assisted Orthopedic Surgery F. 3D templating and patient-specific cutting guides (Knee-Plan) in total knee arthroplasty: postoperative CT-based assessment of implant positioning. Orthop Traumatol Surg Res. 2014;100(6 Suppl):S281–6.
- Hu Y, Li H, Qiao G, Liu H, Ji A, Ye F. Computer-assisted virtual surgical procedure for acetabular fractures based on real CT data. Injury. 2011;42(10):1121–4.
- Issa K, Rifai A, McGrath MS, Callaghan JJ, Wright C, Malkani AL, et al. Reliability of templating with patient-specific instrumentation in total knee arthroplasty. The Journal of Knee Surgery. 2013;26(6):429–33.
- Kerens B, Boonen B, Schotanus M, Kort N. Patient-specific guide for revision of medial unicondylar knee arthroplasty to total knee arthroplasty: beneficial first results of a new operating technique performed on 10 patients. Acta Orthopaedica. 2013;84(2):165–9.
- Leeuwen JA, Grogaard B, Nordsletten L, Rohrl SM. Comparison of planned and achieved implant position in total knee arthroplasty with patient-specific positioning guides. Acta Orthopaedica. 2015;86(2):201–7.
- Pietsch M, Djahani O, Hochegger M, Plattner F, Hofmann S. Patient-specific total knee arthroplasty: the importance of planning by the surgeon. Knee Surg Sports Traumatol Arthrosc. 2013;21(10):2220–6.
- Schweizer A, Furnstahl P, Nagy L. Three-dimensional correction of distal radius intra-articular malunions using patient-specific drill guides. Journal of Hand Surgery - American Volume. 2013;38(12):2339–47.
- Shen F, Chen B, Guo Q, Qi Y, Shen Y. Augmented reality patient-specific reconstruction plate design for pelvic and acetabular fracture surgery. Int J Comput Assist Radiol Surg. 2013;8(2):169–79.
- Small T, Krebs V, Molloy R, Bryan J, Klika AK, Barsoum WK. Comparison of acetabular shell position using patient specific instruments vs. standard surgical instruments: a randomized clinical trial. J Arthroplasty. 2014;29(5):1030–7.
- Victor J, Premanathan A. Virtual 3D planning and patient specific surgical guides for osteotomies around the knee: a feasibility and proof-of-concept study. Bone & Joint Journal. 2013;95-B(11 Suppl A):153–8.
- Vlachopoulos L, Schweizer A, Graf M, Nagy L, Furnstahl P. Three-dimensional postoperative accuracy of extra-articular forearm osteotomies using CT-scan based patient-specific surgical guides. BMC Musculoskelet Disord. 2015;16:336.
- Desender L, Rancic Z, Aggarwal R, Duchateau J, Glenck M, Lachat M, et al. Patient-specific rehearsal prior to EVAR: a pilot study. Eur J Vasc Endovasc Surg. 2013;45(6):639–47.
- Willaert W, Aggarwal R, Harvey K, Cochennec F, Nestel D, Darzi A, et al. Efficient implementation of patient-specific simulated rehearsal for the carotid artery stenting procedure: part-task rehearsal. Eur J Vasc Endovasc Surg. 2011;42(2):158–66.
- Qiu TM, Zhang Y, Wu JS, Tang WJ, Zhao Y, Pan ZG, et al. Virtual reality presurgical planning for cerebral gliomas adjacent to motor pathways in an integrated 3-D stereoscopic visualization of structural MRI and DTI tractography. Acta Neurochir (Wien). 2010;152(11):1847–57.
- Hislop SJ, Hedrick JH, Singh MJ, Rhodes JM, Gillespie DL, Johansson M, et al. Simulation case rehearsals for carotid artery stenting. Eur J Vasc Endovasc Surg. 2009;38(6):750–4.
- Willaert WI, Aggarwal R, Van Herzeele I, Plessers M, Stroobant N, Nestel D, et al. Role of patient-specific virtual reality rehearsal in carotid artery stenting. Br J Surg. 2012;99(9):1304–13.
- Makiyama K, Yamanaka H, Ueno D, Ohsaka K, Sano F, Nakaigawa N, et al. Validation of a patient-specific simulator for laparoscopic renal surgery. Int J Urol. 2015;22(6):572–6.
- Ng I, Hwang PY, Kumar D, Lee CK, Kockro RA, Sitoh YY. Surgical planning for microsurgical excision of cerebral arterio-venous malformations using virtual reality technology. Acta Neurochir (Wien). 2009;151(5):453–63; discussion 63.
- Ferrari V, Carbone M, Cappelli C, Boni L, Melfi F, Ferrari M, et al. Value of multidetector computed tomography image segmentation for preoperative planning in general surgery. Surgical Endoscopy. 2012;26(3):616–26.
- Radecka E, Brehmer M, Holmgren K, Palm G, Magnusson P, Magnusson A. Pelvicaliceal biomodeling as an aid to achieving optimal access in percutaneous nephrolithotripsy. J Endourol. 2006;20(2):92–101.
- van Steenberghe D, Glauser R, Blomback U, Andersson M, Schutyser F, Pettersson A, et al. A computed tomographic scan-derived customized surgical template and fixed prosthesis for flapless surgery and immediate loading of implants in fully edentulous maxillae: a prospective multicenter study. Clin Implant Dent Relat Res. 2005;7 Suppl 1:S111–20.
- Li C, Yang M, Xie Y, Chen Z, Wang C, Bai Y, et al. Application of the polystyrene model made by 3-D printing rapid prototyping technology for operation planning in revision lumbar discectomy. Journal of Orthopaedic Science. 2015;20(3):475–80.
- Leong JL, Batra PS, Citardi MJ. Three-dimensional computed tomography angiography of the internal carotid artery for preoperative evaluation of sinonasal lesions and intraoperative surgical navigation. Laryngoscope. 2005;115(9):1618–23.
- Lu S, Xu YQ, Zhang YZ, Xie L, Guo H, Li DP. A novel computer-assisted drill guide template for placement of C2 laminar screws. Eur Spine J. 2009;18(9):1379–85.
- Kanzaki M, Maeda H, Wachi N, Kikkawa T, Komine H, Isaka T, et al. Complete video-assisted thoracoscopic multi-subsegmentectomy based on patients' specific virtual 3-D pulmonary models. Asian J Endosc Surg. 2013;6(2):110–5.
- Isotani S, Shimoyama H, Yokota I, China T, Hisasue S, Ide H, et al. Feasibility and accuracy of computational robot-assisted partial nephrectomy planning by virtual partial nephrectomy analysis. Int J Urol. 2015;22(5):439–46.
- Kusaka M, Sugimoto M, Fukami N, Sasaki H, Takenaka M, Anraku T, et al. Initial experience with a tailor-made simulation and navigation program using a 3-D printer model of kidney transplantation surgery. Transplantation Proceedings. 2015;47(3):596–9.
- Mandel M, Amorim R, Paiva W, Prudente M, Teixeira MJ, Andrade AF. 3D preoperative planning in the ER with OsiriX: when there is no time for neuronavigation. Sensors. 2013;13(5):6477–91.
- Li H, Wan F, Li J, Sheng L, Li G, Chen G, et al. Flat Detector Computed Tomography-Based “Dual Vessel Fusion” Technique for Diagnosis and Surgical Planning in the Management of Dural Arteriovenous Fistula. World Neurosurg. 2015;84(2):520–7.
- Carter FJ, Schijven MP, Aggarwal R, Grantcharov T, Francis NK, Hanna GB, et al. Consensus guidelines for validation of virtual reality surgical simulators. Simul Healthc. 2006;1(3):171–9.
- Gavazzi A, Bahsoun AN, Van Haute W, Ahmed K, Elhage O, Jaye P, et al. Face, content and construct validity of a virtual reality simulator for robotic surgery (SEP Robot). Ann R Coll Surg Engl. 2011;93(2):152–6.
- collaboration C. Cochrane collaboration's tool for assessing risk bias. Available from: http://handbook.cochrane.org/chapter_8/table_8_5_a_the_cochrane_collaborations _tool_for_assessing.htm.
- Wells GS, B; O'Connell, D; Peterson, J; Welch, V; Losos, M; Tugwell, P. The Newcastle-Ottawa Scale (NOS). 2014.
- Chan K, Bhandari M. Three-minute critical appraisal of a case series article. Indian J Orthop. 2011;45(2):103–4.
- AlAli AB, Griffin MF, Butler PE. Three-Dimensional Printing Surgical Applications. Eplasty. 2015;15:e37.
- Pratt R, Deprest J, Vercauteren T, Ourselin S, David AL. Computer-assisted surgical planning and intraoperative guidance in fetal surgery: a systematic review. Prenat Diagn. 2015;35(12):1159–66.
- See KW, Chui KH, Chan WH, Wong KC, Chan YC. Evidence for Endovascular Simulation Training: A Systematic Review. Eur J Vasc Endovasc Surg. 2016;51(3):441–51.
- Chambers D, Rodgers M, Woolacott N. Not only randomized controlled trials, but also case series should be considered in systematic reviews of rapidly developing technologies. Journal of Clinical Epidemiology. 2009;62(12):1253–60 e4.
- Dalziel K RA, Stein K, Garside R, Castelnuovo E, Payne L. Do the findings of case series vary significantly according to methodological characteristics? Health Technol Assess. 2005;9:iii-iv(2):1-146.
- Stein K, Dalziel K, Garside R, Castelnuovo E, Round A. Association between methodological characteristics and outcome in health technology assessments which included case series. International Journal of Technology Assessment in Health Care. 2005;21(3): 277–87.
- Reeves BC VK, Tsang VTC, Bennett-Lloyd BD, O'Riordan PA, editor A systematic review of case series of paediatric cardiac surgery. 8th Annual Cochrane Colloquium; 2000; Cape Town, South Africa.
- Linde K, Scholz M, Melchart D, Willich SN. Should systematic reviews include non-randomized and uncontrolled studies? The case of acupuncture for chronic headache. Journal of Clinical Epidemiology. 2002; 55(1):77–85.
- IntHout J, Ioannidis JP, Borm GF, Goeman JJ. Small studies are more heterogeneous than large ones: a meta-meta-analysis. Journal of Clinical Epidemiology. 2015;68(8):860–9.
- Cochrane Handbook. Available from: http://handbook.cochrane.org/.