Abstract
Neural substrates of action to the object or this specific direct route, however, remain unclear, especially for the connection from the visual pathway to the motor cortex. The study examined this issue by conducting an fMRI experiment, in which two action generation tasks involving pictures of real objects (PA) and the object’s nouns (NA) were used, with pictures naming (PN) and covert noun reading (NR) being the control tasks. The result showed that the model predefined for the PCC and precuneus connecting IPL to the posterior-medial frontal cortex dominated over the others (with 0.45 probability), suggesting that the PCC and the precuneus locate at the neural substrates of action to the object. Furthermore, a feasibility study suggests that the neural pathway composed of the V3/MT, precuneus, PCC, and PM (premotor cortex) forms the direct route from perception to action, which also links to the dorsal pathway so that the perception of objects bypasses the semantic ventral pathway and then directly cues actions via the affordance.
1. Introduction
Motor system in the brain is activated during observing the manipulable objects and the relationship between an object and potential actions associated with the object that is called as “affordance” [Citation1,Citation2]. The motor system has been found to be involved in processing names of graspable objects [Citation3,Citation4]. Not only object nouns, but also object pictures [Citation5] evokes the associated motor codes and modulate motor responses. Recent findings from behavioral studies showed the insignificantly different affordance effects evoked by object pictures and nouns [Citation6], but the underlying neural mechanism is not clear yet.
For the action generation pathway from an object, the action knowledge of an object was suggested to be retrieved through a non-semantic “direct visual route” from vision to action [Citation7–9]. In their case study of the patient TP with left occipito-temporal brain damage, the result that action naming was spared relative to object naming [Citation8,Citation9] showed that action programs could be accessed through a direct visual route that bypasses a semantic route. Furthermore, from previous studies, the dissociation between object naming and appropriate action to object generation was found [Citation10]. These studies also revealed the existence of the “direct visual route” which challenges the traditional view that semantic knowledge is the only pathway from vision to action.
The “direct visual route” could be an alternative route of accessing actions for objects, but has been questioned to be involved in retrieving actions for nouns. Riddoch [Citation7] pointed out that a patient with lesions in the left parietal cortex was not able to generate appropriate right-hand actions toward an object even though his semantic knowledge was preserved, which was attributed to the deficit in the direct visual route, but he was able to perform hand gestures when he was given the object’s nouns. Their result suggested that the direct visual route contributed to actions related to objects and wasnot critical to retrieving actions for nouns, while visual features of an object may be necessary for activating the “direct visual route”. Nevertheless, neural mechanisms that underlie the direct visual route are still unknown.
In this fMRI study, by involving both object pictures and nouns in the tasks of object naming, noun reading and action generation, we investigated the similarities and differences in the neural substrates that mediate action generation from object pictures and corresponding nouns to identify cortical regions that are crucial to the direct route from vision to action [Citation7]. Object naming and noun reading were involved in the control of the neural processes that were specific to each stimulus modality (pictures and nouns) but unrelated to action retrieval and generation. Then, it is expected that action generation from both object pictures and nouns would engage the motor-related cortical regions [Citation11]. Furthermore, some direct evidence for some cortical regions that were critical to the direct visual route is expected to be found.
Dynamic causal modeling (DCM) [Citation12] on fMRI time series during the action generation from object pictures for the direct evidence of the direct visual route between the frontal area and the posterior area is used. By building DCM models for different connection hypotheses and comparing them, the best hypothesis can be selected as evidence.
2. Methods
2.1. fMRI data acquisition
MRI data was acquired by a 3-T GE MRI scanner (GE Healthcare, Waukehsa, U.S.A.). and functional data were obtained using an echoplanar imaging (EPI) sequence for further analysis. The scanning parameters were as follows: repetition time (TR)=2000 ms; echo time (TE)=30 ms; field of view = 220 × 220mm2; number of slices = 37; slice thickness = 3.4375 mm; voxel size = 3 × 3×3.4375 mm3. Totally, there were 648 EPI images obtained in 4 runs. A T1 image was also obtained using the 3 D FSPGR sequence with the voxel size of 1 × 1×1 mm3.
2.2. Subjects
Sixteen right-handed native Chinese speakers participated in this experiment (age range: 20–25, 11 males and 5 females), and none of the subjects had a history of psychiatric or neurological illness. Furthermore, all subjects signed informed consent before the experiment, and the experimental procedures were approved by the ethics committee of Tongji University.
2.3. fMRI paradigm
Subjects performed two action generation tasks in which subjects responded to objects and their nouns with appropriate hand gestures, and two control tasks in which subjects named the object pictures or read the object nouns in a single imaging session. The experimental procedure was programmed and presented via the Presentation Software.
Block-designs were used for all the tasks. In the task of action generation from objects, subjects were asked to mime an action corresponding to an object picture (PA). In the control task, subjects silently named the same object picture (PN). In the third task, subjects mimed an action related to an object’s noun (NA). Same nouns were read silently in the noun reading (NR) task which was designed as a control task. Both control tasks were intended to control for visual inputs. After the scanning session, the subjects were asked to recall the actions which they had performed when being given the object pictures and nouns.
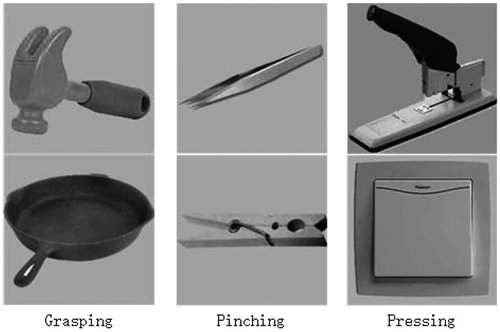
Subjects chose from one of the following three hand movements in response to a picture or a word: grasping, pressing and pinching. In the PA, subjects were asked to covertly generate one of these three verbs congruent with the object use. In the NA, subjects performed the corresponding action using the right hand without moving the arm.
A pilot rating study was carried out to select appropriate stimuli from 74 candidate objects. A stimulus was selected here with high scores of naming consistency, action consistency and familiarity. The scores of naming consistency and action consistency were the frequency of the most common response divided according to the frequency of the second-most frequent response. The score of familiarity was the average score of the participants’ responses (0–7). 24 stimuli were chosen with the naming consistency: 10.125, the related action consistency: 9.57 (at least 87% answers were the same) and the familiarity: 5.44. These stimuli were classified averagely as three types corresponding to their related actions (grasping, pressing and pinching), and all of them were gray scale images with the same size. Pictures of the stimuli are shown in .
The object pictures used in the PA and the PN were in one-to-one correspondence with the nouns used in the NA and the NR. For example, a hammer was shown in the PA, and correspondingly, a noun “chuizi” (“hammer” in Chinese) was shown in the NA.
The order of the tasks was counter-balanced across subjects. Stimuli and block orders were also pesudo-randomized in each run. The stimuli of object pictures were grayscale images with the same size presented in a light-gray background. A video projector was employed to project stimuli onto a mirror mounted above the head coil, and the stimuli were visible through this mirror. Before each task, a visual cue for the coming task (for two seconds) was presented. There were 4 runs in the experimental session. To be specific, for each run, eight blocks of three different tasks (including a control task) and rest twelve blocks were to be submitted. Each task block was composed of six trials, each of which involved stimulus presentation for 1500 ms followed by a fixation cross for 1000 ms. Each rest block was interspersed between two task blocks and lasted for 10 s, in which subjects were asked to fixate on a cross at the screen center. In the scanning session, totally 240 volumes were involved.
2.4. Analysis on response results
For the PA and NA tasks, the subjects’ responses in their memory were recorded immediately after the experiment. The repeated measure ANOVA was calculated to find if significant stimuli effect existed in the PA and the NA.
2.5. fMRI data analysis
FMRI data was analyzed using the SPM8 (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (Mathworks, Natick, MA). EPI images were realigned, co-registered and normalized on the Montreal Neurological Institute (MNI) EPI template, and they were also smoothed using an 8 mm FWHM kernel in the preprocessing step. Then, two steps of statistical analysis came after the preprocessing step.
In the first step, a general linear model (GLM) was adopted for the individual statistical analysis. For each subject, the GLM was employed to estimate contrast images, indicating the brain activation in (1) contrasting activation during the action generation process in the PA comparative to picture naming (PA-PN); (2) noun-action semantic association, while controlling noun reading and comprehension, by contrasting activation during the NA relative to noun reading (NA-NR). All the contrast images were calculated by conducting the paired T test. In this step, the BOLD signal in each run was modeled by convolving the onsets of all blocks with a canonical hemodynamic response function (HRF).
In the second step, group statistical analysis (random effect analysis) was implemented by combining the contrast images acquired in the previous step. Contrast images were obtained using T contrasts (p < .0001 voxel level) with cluster-level family-wise error (FWE) correction (p < .05, corrected).
After the activations of all contrast images were obtained, firstly images were analyzed contrastively for each task, and we also checked whether the activation results conformed to those reported in previous work. Then a direct comparison between PA-PN and NA-NR was conducted to locate the locations of significant different activities, and it was calculated by T contrasts (p < .0005 at voxel level) and cluster-level family-wise error (FWE) correction (p < .05, corrected). The next step was to identify the cortical regions activated by action generation tasks and examine the regions activated by PA-PN and NA-NR. Finally, the activations in the whole brain were compared, and at the same time, the similarities and differences in the cortical regions, especially the motor system were analyzed.
The DCM was conducted to estimate and make inferences about casual relations among brain areas as well as how the relations is modulated by experimental tasks [Citation12]. The DCM for fMRI combines a bilinear model of neural dynamics with an empirical hemodynamic model that describes the transformation of neuronal activity into BOLD response [Citation13].
The DCM based on our real fMRI data is used in SPM12 (Statistical Parametric Mapping, www.fil.ion.ucl.ac.uk/spm). DCMs were constructed for each single subject separately. The aim of our DCM analysis was to find the best hypothesis for the connections among frontal areas (PM), posterior areas (IPL) and the precuneus/PCC and offer the direct evidence of whether the precuneus/PCC involves a pathway from posterior areas to posterior areas.
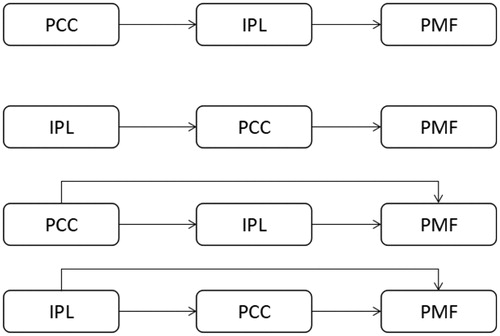
Three VOIs of the left premotor area, the precneus/PCC (PCC region) and the left IPL were extracted according to the activations of PA and NA tasks. According to the dorsal stream from posterior cerebrum to the frontal cerebrum, we decided to just compare the unidirectional models in order to selective analyze the information stream from vision to action. Four DCM models on the PA task of the three VOIs were hypothesized to find the connections ().
3. Results
3.1. Behavioral results
The mean accuracy of subjects’ responses in the two tasks was 92%. The mean accuracy of a single task was and for the PA and the NA respectively. The accuracy was high and above the related action consistency, which showed that the responses of subjects were also significantly consistent. A repeated measures ANOVA revealed an insignificant effect of the stimuli (F(1,15)=0.147; p>.05). The responses of the two tasks (PA and NA) were similar.
3.2. Brain activation for PA-PN and NA-NR
Activation for noun-related affordance (NA-NR) was shown (; ) in the left precentral/postcentral gyrus, left inferior/middle frontal gyrus (IFG/MFG), left mid-cingulate cortex, left middle temporal gyrus and left inferior parietal lobule. Object-related affordance (PA-PN) (; ) activated the bilateral postcentral gyrus, left precentral gyrus, left inferior frontal gyrus (IFG), bilateral middle/superior frontal gyrus (MFG/SFG) and the left inferior parietal lobule (IPL). PA-PN and NA-NR activated cortical regions including the left precentral and postcentral gyrus, left superior/middle/inferior frontal gyrus, and left inferior parietal lobule, consistent with the previous results of action execution, action miming and verb generation [Citation14,Citation15]. However, PA-PN showed greater activation than NA-NR in the left precentral and postcentral gyrus, left inferior parietal lobule and superior/middle frontal gyrus.
Table 1. Activation peaks and extents for NA-NR.
Table 2. Activation peaks and extents for PA-PN.
3.3. Comparison between PA-PN and NA-NR
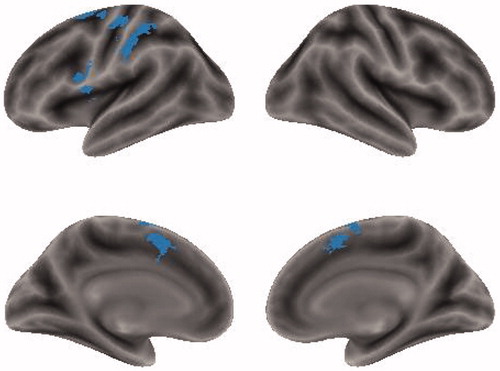
The statistical map () for the direct comparison of the two tasks ([PA-PN]-[NA-NR]) was calculated. The bilateral preceneus and bilateral posterior cingulate cortices (PCC) showed significant difference (), but the reverse contrast ([NA-NR]-[PA-PN]) showed no significant activation.
Figure 3. Activation maps for PA-PN and NA-NR. Activations for PA-PN (a) were located bilaterally. Activations for NA-NR (b) were located mostly in the left hemisphere. The activated regions for NA-NR were subsets of those for PA-PN.
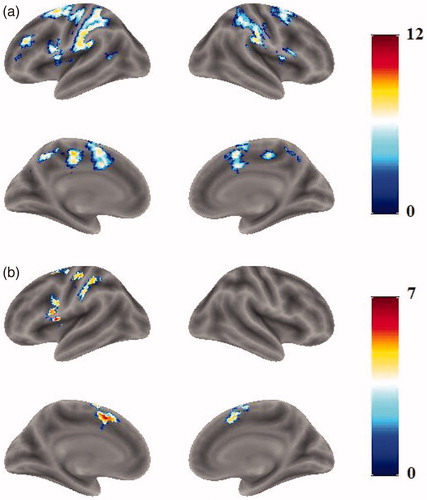
Table 3. Activation peaks and extents for the [PA-PN]-[NA-NR] contrast.
3.4. Comparison between PA-PN and NA-NR
The conjunction analysis on PA-PN and NA-NR was performed to identify cortical regions shared by PA-PN and NA-NR. Then, overlapped activation was found in the left inferior frontal gyrus, bilateral supplementary motor area (SMA), left pre/postcentral gyrus, left inferior parietal lobule and left anterior cingulate gyrus (). Moreover, the activated regions for NA-NR were subsets of those for PA-PN.
3.5. Dynamic causal modelling
demonstrates the pattern of endogenous connections and modulations in the DCM network. The endogenous connections among PCC, IPL and PMF were different in models. The endogenous connection is switched on only when this connection exists in the causal model. The modulations of PA were set on the intrinsic connections in each model.
Figure 6. Model specification. The models have the different endogenous connectivity, and the modulations of PA were implemented in the endogenous connections. All of the three ROIs were deemed as the source of models.
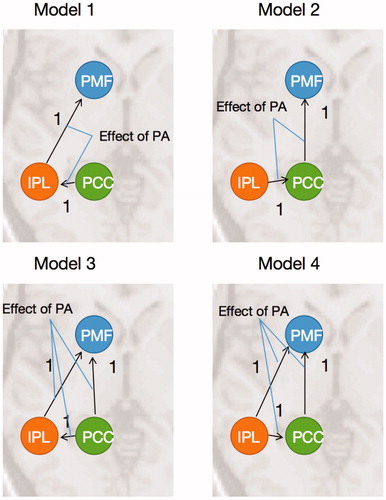
The models have different endogenous connections, but the same input in all models. The driving inputs of PA were set to all the ROIs (IPL, PCC and PMF), because there could be some other stimuli effect these ROIs as inputs.
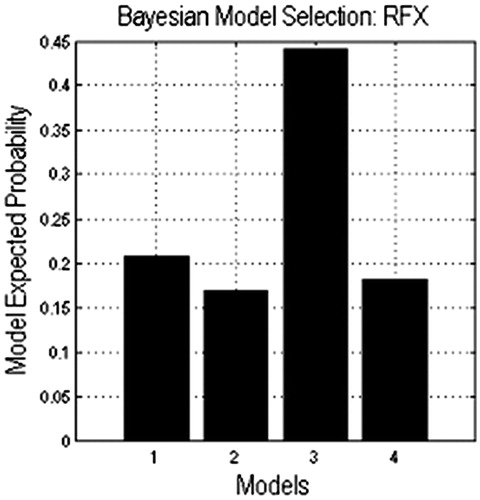
All of the models were compared, and it was found that model 3 got the highest model expected probability (). The expected probability of model3 is about 0.45, but the expected probability of the other models didn’t reach 0.25, which means that in the task of action generation from object pictures, two parallel causal relationships existed from the PCC to the PM.
4. Discussion and conclusion
4.1. Summary
In this study, brain activations for generating actions from object pictures and nouns were compared to examine their shared and distinct neural substrates. To this end, fMRI experiments involving action generation tasks with object pictures (PA) and nouns (NA) and two control tasks: picture naming (PN) and noun reading (NR) were performed. Conjunction analysis revealed that PA-PN and NA-NR shared activations in the left IFG, the bilateral SMA, the left pre/postcentral gyrus and the left IPL. The comparison between PA-PN and NA-NR failed to show significant activations in the motor system. However, higher activation was found for PA-PN than for NA-NR in the bilateral precuneus and PCC.
4.2. A feasibility study: Neural substrates for the direct route from vision to action
Perhaps the most important finding in this study was the results from the comparison between PA-PN and NA-NR. To be specific, significant activations in the Precuneus and PCC were found. In our results, PA-PN showed greater activation than NA-NR in the precuneus and PCC. Why do the precuneus and PCC activate? It was a question, and the model comparison of the DCM could give an explanation.
Model 3 got the highest model expected probability in the comparison results of four models, which might mean that the precuneus/PCC connected and transferred some information to the IPL and the PM concurrently. Furthermore, the IPL connected and transferred some information to the PM. However, what the functions of these regions were and what the information among these regions was are still unclear.
Previous studies have showed the activations in the bilateral precuneus and the bilateral PCC for the action generation tasks [Citation16,Citation17], which are consistent with our results. It has been indicated that the Precuneus is involved in visuospatial and motor imagery [Citation18,Citation19] while the PCC encodes motor episodic memory, spatial relationships and self-referential mental thoughts [Citation20–22]. The previous result suggested that PCC regions may play crucial roles in extracting visual-motor features from objects for the purpose of action generation. The previous finding of the dissociation between the visual-motor pathway (direct visual route) and the semantic pathway [Citation7,Citation9], together with our DCM results, led to the implication that the precuneus/PCC could be involved specifically for the direct visual route from vision to action with the function of extracting visual-motor and spatial relationship features.
The PCC region has also been thought to be the convergence node between the posterior cerebral regions and the frontal cortex [Citation23,Citation24]. With our results obtained from direct comparison analysis and DCM results, the precuneus/PCC and the frontal cortex with different levels of activation in PA-PN and NA-NR could be attributed to the strong connection from the PCC to the frontal cortex (anterior parts) in action generation from object pictures but a significantly weaker connection for action generation from object nouns. These results showed that in the action generation from object pictures, the precuenus/PCC transferred the visual and spatial information to the PM facilitating the action execution. Besides, the visual and spatial information was transferred to the IPL region in which visual features were analyzed for planning and monitoring the execution of suitable actions [Citation25].
In conclusion, it is shown that the neural mechanisms for action control, planning and intention processing within the frontal-parietal motor networks are engaged in action generation tasks irrespective of the modality of the visual input. Most importantly, the PCC and precuneus are involved specifically for action generation from object pictures and associated with the “direct visual route” from vision to action which has been proposed in previous research. Moreover, the contribution of the PCC and precuneus to the direct visual route is 2-fold: firstly, they extract episodic motor experiences of object-hand interactions and elicit motor simulations from visual features of objects; secondly, they work as bridge to connect the posterior cortex and the frontal cortex and help the PM transform visual-spatial information of input into motor codes. Finally, our results show that the visual direct route could be a branch of dorsal stream starting from the PCC.
Additional information
Funding
References
- Rizzolatti G, Camarda R, Fogassi L, et al. Functional organization of inferior area 6 in the macaque monkey. Exp Brain Res. 1988;71:491–507.
- Murata A, Fadiga L, Fogassi L, et al. Object representation in the ventral premotor cortex (area F5) of the monkey. J Neurophysiol. 1997;78:2226–2230.
- Bub DN, Masson ME, Cree GS. Evocation of functional and volumetric gestural knowledge by objects and words. Cognition 2008;106:27–58.
- Marino BF, Gough PM, Gallese V, et al. How the motor system handles nouns: a behavioral study. Psychol Res. 2013;77:64–73.
- Péran P, Cardebat D, Cherubini A, et al. Object naming and action-verb generation in Parkinson's disease: a fMRI study. Cortex. 2009;45:960–971.
- Tucker M, Ellis R. Action priming by briefly presented objects. Acta Psychol. 2004;116:185–203.
- Riddoch MJ, Humphreys GW, Price CJ. Routes to action: Evidence from apraxia. Cogn Neuropsychol. 1989;6:437–454.
- Yoon EY, Heinke D, Humphreys GW. Modelling direct perceptual constraints on action selection: The Naming and Action Model (NAM). Vis Cogn. 2002;9:615–661.
- Yoon EY, Humphreys GW, Riddoch MJ. Action naming with impaired semantics: neuropsychological evidencecontrasting naming and reading for objects and verbs. Cogn Neuropsychol. 2005;22:753–767.
- Hodges JR, Bozeat S, Ralph MAL, et al. The role of conceptual knowledge in object use evidence from semantic dementia. Brain. 2000;123:1913–1925.
- Marino BF, Sirianni M, Volta RD, et al. Viewing photos and reading nouns of natural graspable objects similarly modulate motor responses. Front Hum Neurosci. 2014;8:968.
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302.
- Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med. 1998;39:855–864.
- Péran P, Démonet J, Cherubini A, et al. Mental representations of action: the neural correlates of the verbal and motor components. Brain Res. 2010;1328:89–103.
- Binkofski F, Buccino G, Posse S, et al. A fronto‐parietal circuit for object manipulation in man: evidence from an fMRI‐study. Eur J Neurosci. 1999;11:3276–3286.
- Allendorfer JB, Lindsell CJ, Siegel M, et al. Females and males are highly similar in language performance and cortical activation patterns during verb generation. Cortex 2012;48:1218–1233.
- Karunanayaka P, Schmithorst VJ, Vannest J, et al. A group independent component analysis of covert verb generation in children: a functional magnetic resonance imaging study. Neuroimage 2010;51:472–487.
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583.
- Kawashima R, Roland PE, O'Sullivan BT. Functional anatomy of reaching and visuomotor learning: a positron emission tomography study. Cereb Cortex. 1995;5:111–122.
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 2001;104:667–676.
- Wagner AD, Shannon BJ, Kahn I, et al. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453.
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32.
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage 2008;42:1178–1184.
- Pearson JM, Heilbronner SR, Barack DL, et al. Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn Sci. 2011;15:143–151.
- Andersen RA. Inferior parietal lobule function in spatial perception and visuomotor integration. Handb Physiology 1987;5.

![Figure 4. Activations for the [PA-PN]-[NA-NR] contrast, shown in the preceneus and PCC.](/cms/asset/54b7ccfc-7592-402a-8d48-32c78e10248a/icsu_a_1557903_f0004_c.jpg)