Abstract
Surgery is a curative treatment option for many patients with malignant tumors. Increased attention has focused on the combination of surgery with chemotherapy, as multimodality treatment has been associated with promising results in certain cancer types. Despite these data, there remains clinical equipoise on optimal timing and patient selection for neoadjuvant or adjuvant strategies. Radiomics, an emerging field involving the extraction of advanced features from radiographic images, has the potential to revolutionize oncologic treatment and contribute to the advance of personalized therapy by helping predict tumor behavior and response to therapy. This review analyzes and summarizes studies that use radiomics with machine learning in patients who have received neoadjuvant and/or adjuvant chemotherapy to predict prognosis, recurrence, survival, and therapeutic response for various cancer types. While studies in both neoadjuvant and adjuvant settings demonstrate above average performance on ability to predict progression-free and overall survival, there remain many challenges and limitations to widespread implementation of this technology. The lack of standardization of common practices to analyze radiomics, limited data sharing, and absence of auto-segmentation have hindered the inclusion and rapid adoption of radiomics in prospective, clinical studies.
1. Introduction
Cancer is the second leading cause of death worldwide, accounting for an estimated 9.6 million deaths in 2018 [Citation1]. Advances in oncologic treatment, including novel surgical techniques, combined modality approaches and new chemotherapeutic regimens, have led to significant improvements in both cancer-related mortality and disease-free survival in multiple tumor types. Efforts to best identify patients who will respond to and benefit from such therapies remains a critical research priority in oncology.
Chemotherapy and surgery are the mainstay treatments for many tumor types [Citation2,Citation3]. While surgery remains the curative treatment for many solid tumors, combined surgical treatment with chemotherapy has been associated with improved survival and systemic disease control [Citation4,Citation5]. Neoadjuvant chemotherapy, which refers to the administration of systemic treatment before surgery, is routinely administered for inoperable breast, colorectal and lung cancers, and is also an option for other solid tumors [Citation6–9]. Such upfront therapy is intended to reduce the size of the tumor and control progression of disease. Downstaging the tumor with upfront systemic therapy may increase margin-negative resections, make previously inoperable tumors resectable, and help manage micro-metastatic disease [Citation10–12]. Adjuvant chemotherapy is provided after surgical intervention and is designed to reduce recurrence of disease [Citation13]. Adjuvant chemotherapy is routinely used in high-risk patients with breast, colon, testicular, ovarian, lung, and pancreatic cancers [Citation14].
As there are risks associated with both neoadjuvant and adjuvant chemotherapy, efforts to determine which patients may benefit from treatment are warranted. For neoadjuvant chemotherapy, progression of disease or development of metastasis may preclude surgical intervention, rendering the disease harder to treat [Citation15]. Additionally, side effects from neoadjuvant therapy may leave a patient unfit for surgery. Adjuvant chemotherapy may similarly lead to long term complications due to the side effects from prolonged treatment [Citation16]. Identifying predictors of treatment effectiveness would not only improve patient selection but also optimize the timing of these treatments.
Evaluation of imaging modalities like computed tomography (CT), contrast enhanced ultrasound, magnetic resonance imaging (MRI), and positron emission tomography (PET) to determine chemotherapy response of patients in different studies have given inconsistent results [Citation17,Citation18]. Analysis in these studies relied on either the sole expertise of radiologists or limited quantitative imaging parameters [Citation17,Citation18]. While radiologists have the training and ability to discern many imaging features, microscopic details relevant to clinical outcomes may be missed due to the limitations in human visualization [Citation19].
Radiomics is an emerging and ever evolving field of study involving the extraction of many advanced quantitative features from radiographic images [Citation20]. Computer algorithms are used to mine these features by analyzing multiple aspects of the images (i.e. physical, textural, histogram, filter-based, and fractal) [Citation20]. Radiomics have been used to characterize tumor phenotypes for predicting prognosis and therapeutic response for various diseases [Citation21,Citation22]. Radiomics serve as a diagnostic and prognostic tool, which can be applied at many different timepoints in oncologic care. Use of this technology in the diagnosis stage, treatment planning stage, and surveillance stage (as outlined below in ) can complement traditional oncologic care and help personalize treatment options for patients with solid tumors. In this paper, we analyze and summarize papers that use radiomics to predict prognosis, recurrence, survival, and therapeutic response to chemotherapy in both neoadjuvant and adjuvant settings. Such data can be leveraged to appropriately identify patients who may benefit from perioperative chemotherapy and help optimize timing of surgery.
Figure 1. This graphic gives a patient a top-level view of their progression from an initial screening, chemotherapy, surgery, and surveillance after resection. Radiomics serve as a diagnostic and prognostic tool, which can be applied at many different timepoints in oncologic care. Use of this technology in the diagnosis stage, treatment planning stage, and surveillance stage can complement traditional oncologic care and help personalize treatment options for patients with solid tumors [Citation23].
![Figure 1. This graphic gives a patient a top-level view of their progression from an initial screening, chemotherapy, surgery, and surveillance after resection. Radiomics serve as a diagnostic and prognostic tool, which can be applied at many different timepoints in oncologic care. Use of this technology in the diagnosis stage, treatment planning stage, and surveillance stage can complement traditional oncologic care and help personalize treatment options for patients with solid tumors [Citation23].](/cms/asset/8bb2032b-511e-4e05-bbe5-938d387af85f/icsu_a_1994014_f0001_c.jpg)
2. Technical approaches
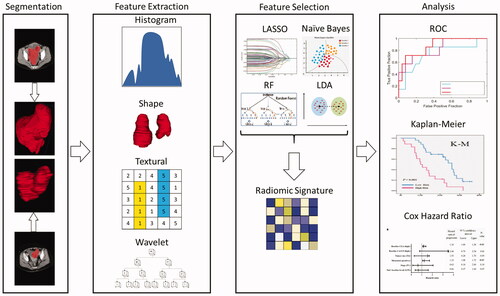
Radiomics is an advanced computational method that derives radiological features from medical images with the ability to identify, diagnose, and predict patient response for various diseases. Radiomics can be derived explicitly using so-called handcrafted features, or implicitly using deep learning approaches. The general workflow of radiomics involves four main components: image acquisition, tumor segmentation, radiomic signature, and machine learning analysis. Each aspect of the workflow is complex, and the implementation varies across institutions and research groups. gives a general overview of the radiomic process from patient to predicted outcome.
Figure 2. This flowchart shows the generalized overview of the neoadjuvant and adjuvant studies from patient image acquisition (CT, MRI, PET), tumor segmentation, radiomic signature, machine learning analysis, and the predicted outcome. These studies capture prognosis, recurrence, therapeutic response, survival, and tumor volume. These various outcome categories describe different aspects of the effectiveness of either neoadjuvant or adjuvant chemotherapy [Citation24,Citation25].
![Figure 2. This flowchart shows the generalized overview of the neoadjuvant and adjuvant studies from patient image acquisition (CT, MRI, PET), tumor segmentation, radiomic signature, machine learning analysis, and the predicted outcome. These studies capture prognosis, recurrence, therapeutic response, survival, and tumor volume. These various outcome categories describe different aspects of the effectiveness of either neoadjuvant or adjuvant chemotherapy [Citation24,Citation25].](/cms/asset/2f8676dc-ec5c-4d69-a9ed-45e31fad8327/icsu_a_1994014_f0002_c.jpg)
2.1. Patient cohorts
The studies summarized in this paper were identified by combinations of the following search terms on Google: “adjuvant”, “neoadjuvant”, “cancer”, “machine learning”, “chemotherapy”, and “radiomics”. Twelve papers including patients who received neoadjuvant therapy and nine including patients who received adjuvant therapy are included in this review. These particular papers were chosen due to their varying cancer types, depth of knowledge conveyed about machine learning, and thorough understanding of radiomic analysis.
and illustrate the breadth of tumor types included in this review: ovarian, breast, gastric, pancreatic, rectal, gastroesophageal junction, cervical, prostate, sarcoma, mesothelioma, glioblastoma, lung, and skin and soft tissue. While the prognosis and management of each of these tumors is unique, radiomics provide a universal tool to aid in diagnosis and prognosis for these patients. In each study, standard protocols concerning patients and protected health information (PHI) are adhered to, such as ensuring the anonymization of PHI, having accountability to an institutional review board, and including waivers of informed consent. Clinical factors, which vary for each study, are acquired by reviewing patients’ medical records. Patient inclusion and exclusion criteria are given that explain how the final cohort is developed. Each study reviewed patients for a duration of years, with some using a second or third cohort from another window of time as a validation set. Some studies highlighted in this review paper use cohorts from multiple institutions, with one set being the training data, and the other serving as the validation set. All of the performance metrics given in and are derived from the validation sets of each paper.
Table 1. Summary of technical approaches for neoadjuvant studies.
Table 2. Summary of technical approaches for adjuvant studies.
Table 3. Performance metrics from neoadjuvant studies in this review.
Table 4. Performance metrics from adjuvant studies in this review.
2.2. Image acquisition & data processing
Each medical center represented in these studies uses different scanning equipment, parameters, and reconstruction methods in the acquisition of their images. The imaging modalities across the studies vary between computed tomography (CT) with or without contrast, magnetic resonance imaging (MRI) at 1.5 and/or 3.0 Tesla, radiomics of multiparametric MRI (RMM), and fluorodeoxyglucose-positron emission tomography (FDG-PET). The segmentation of tumor regions likewise varies between institutions and studies. Manual segmentation, semi-automatic, and automatic segmentation methods are represented across these studies. All studies have the segmentation results under the supervision of and approved by one or more expert radiologist. Each study scaled and normalized the segmented images and applied dilation or filtration according to their standards. and give the imaging modality used for each study.
2.3. Radiomic analysis
The extraction of radiomic features deviates between each study. The number of initial features range from the hundreds to the thousands. Matlab, the PyRadiomics library in Python, and other in-house software developed by some institutions are used to extract radiomics features from the region of interest. Many different methods of feature selection are used to reduce the number of features. First, the majority of institutions remove duplicate values from the feature list. Most state the exclusion of highly correlated features, as well as grouping highly correlated features together. These groups are identified by a Pearson linear correlation coefficient (varies by study), and all but the most significant features are eliminated. To further reduce the dimensionality of the features, the studies use one or more of these techniques: least absolute shrinkage and selection operator (LASSO), recursive features elimination based on Naive Bayes (RFE-NB), selection by filter based on linear discriminant analysis (SBF-LDA), and recursive features elimination based on random forest (RFE-RF). Final parsing of features is completed by using L1 regularization, Cox modeling, and Mann-Whitney U test. and summarize the radiomic features extracted, selected, and the models used to reduce feature dimensionality. gives a visual representation of a generalized version of image data processing and radiomic analysis done in these studies.
2.4. Prediction modeling
The prediction models created from each study are guided by the desired outcome. The model types can be broken up into four main outcomes - prognosis, recurrence, survival, and therapeutic response. Studies analyzing recurrence or therapeutic response of any kind (ImmunoScore of gastric cancer (ISgc), pathologic complete response (pCR), pathologic good response (pGR), radiomics ImmunoScore (RIS), radiomic score (RS), tumor size) use supervised learning methods [Citation26–28]. Whereas studies analyzing prognosis or survival (disease-free survival (DFS), overall survival (OS), progression-free survival (PFS), recurrence-free survival (RFS)) use unsupervised learning techniques. Common approaches to supervised learning include LASSO-Cox, linear discriminant analysis (LDA), linear regression (LR), multivariate LR (Mult. LR), multivariable modeling (Mult. modeling), random forest (RF), and support vector machines (SVM). Unsupervised learning approaches in these studies include k-means clustering and non-negative matrix factorization (NMF). The prediction modeling algorithms for each study are referenced in and .
Statistical analysis varied between institutional studies. Many studies use the t test, χ2 test, Fisher’s test, or Mann-Whitney U test for comparisons between groups, given the type of variable. All statistical tests are two-sided. A p-value <0.05 is considered significant. Accuracy (Acc), area under the receiver operating characteristic curve (AUC), sensitivity (Sn), and specificity (Sp) are used to evaluate the model performance for therapeutic response from various studies. Kaplan–Meier (KM) curves are used to generate survival curves and compared using a log-rank test. The Cox proportional hazards model is used for univariate and multivariate analysis in many studies to analyze survival with a hazard ratio (HR). Statistical Product and Service Solutions (SPSS), and R software are the primary software packages used for statistical analysis in these studies.
2.5. Deep learning
Deep learning is a recent advancement in artificial intelligence that incorporates many layers of computation to extract high-level features from raw input [Citation47]. Deep learning has been useful in many areas of interest within medical applications such as survival, image segmentation, and classification. These algorithms increasingly are implemented in medical imaging and have great potential for research and as an important tool in clinical radiology [Citation48].
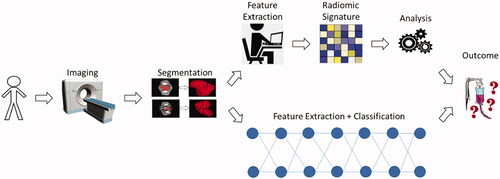
Unlike shallow layered machine learning algorithms that require hard-coded and handcrafted features, deep learning algorithms derive generalized features from learning on a training set. This model established through training is validated on an unseen test set, and gives predictions of the probability of a certain class. Convolutional neural networks (CNNs), stacked denoising autoencoders (SDAs), and deep recurrent neural networks (RNNs) are examples of different types of deep neural networks. gives a top-level overview of the ways radiomics can be implemented between using handcrafted features and machine learning versus implicitly with deep learning.
Figure 4. This flowchart shows the generalized overview of the neoadjuvant and adjuvant studies from patient image acquisition (CT, MRI, PET), tumor segmentation, radiomic signature, machine learning analysis, and the predicted outcome in the traditional machine learning radiomics approach versus the deep learning approach.
One drawback of deep learning is that these algorithms need a vast amount of data to detect a reliable pattern for successful predictions and regression outcomes. Individual medical centers may not have the appropriate data needed to implement these algorithms. Transfer learning, data augmentation, and regularization techniques are some methods used to overcome the dearth in available data.
3. Clinical applications
In the previous section, various technical approaches used to develop a radiomics model are summarized. This section describes the clinical applications of radiomics and highlights some of the performance metrics from the aforementioned studies. These compiled performance metrics do not describe the full range of results for each study, but a succinct picture of comparable performance metrics. Many of these studies combine their radiomic features with clinical and other quantitative features. These results report solely the radiomic feature results from these studies for simplicity. We do not attempt to analyze any of the data listed in this section, but show the range of metrics to give a sense of typical results from these types of studies. and show the performance metrics for the radiomics studies highlighted in this review paper, separating the studies that focused on neoadjuvant chemotherapy from the studies that focused on adjuvant chemotherapy.
3.1. Improved tumor detection
Traditional imaging modalities, including CT and MRI, are critical for the characterization of tumor burden. These imaging modalities are utilized for clinical decision making, treatment planning, and prognosis. Despite high resolution images, small metastatic deposits may be missed. Clinically, detection of these micrometastases may impact surgical planning or treatment course. By using texture analysis to assess subtle changes in tissue, radiomics may allow for improved detection of early micrometastatic disease. One study, published in 2018, tested this hypothesis through evaluation of a mouse model in colorectal cancer. In this study, three independent texture features were associated with later appearance of metastatic deposits on MRI, thus suggesting that early tumor detection may be possible using this technique [Citation49]. While many studies have focused on utilization of radiomics for distinguishing benign and malignant lesions or assessing therapy response, such data are promising as they suggest a role for radiomics in initial diagnosis.
3.2. Predicting response after neoadjuvant therapy
Appropriate selection of patients for neoadjuvant therapy remains a top research priority for a myriad of oncologic diseases. The intent of neoadjuvant therapy is to decrease tumor size, control micro-metastatic disease, and allow for appropriate patient selection for surgery. Radiomics have been studied as a tool for the a priori identification of patients who would benefit from such upfront chemotherapy. For patients with gastric cancer, for example, radiomics have been utilized to accurately predict response to systemic therapy. A recent study, including 106 patients with neoadjuvant chemotherapy before gastrectomy, introduced a CT-based radiomics score to predict response. The published” radclinicalscore” incorporated clinical variables and radiomic features, and was demonstrated to be highly effective at predicting treatment responders (AUC 0.77 in the training cohort, and AUC 0.82 in the validation cohort) [Citation50].
In some tumors, such as locally advanced rectal cancer, pathologic complete response is possible after upfront chemoradiotherapy. As such, identification of treatment responders may obviate the need for resection in these patients. Notably, use of total neoadjuvant therapy (TNT) has become standard of care at many institutions, given the results of large-scale clinical trials in locally advanced rectal cancer [Citation51]. Given this paradigm shift in rectal cancer treatment, new methods to predict pathologic complete response in this patient population have been developed [Citation52]. Similarly, pretreatment radiomic features have been shown to predict pathologic response after neoadjuvant therapy in patients with non-small cell lung cancer and breast cancer [Citation53,Citation54]. These noninvasive proxies for tumor pathology have the potential to revolutionize locoregional options for cancer treatment and potentially avoid unnecessary surgery in treatment responders.
3.3. Selecting patients for adjuvant therapy
In the adjuvant setting, radiomics can be utilized to help clinicians prognosticate after surgery and determine which patients may require more aggressive treatment or follow-up. The integration of radiomics into clinical risk scores may improve risk stratification of patients and selection of patients who would benefit from adjuvant therapy after tumor resection. Such models have been successfully developed and validated in lung cancer [Citation46]. While half of the adjuvant AUC results in this study range from 0.66 to 0.79, with the other half in the 0.80 s (excluding an outlier of 0.47), the hazard ratios for the adjuvant studies do not reflect the fullness of each study, as they often are compared to patients under varying circumstances (i.e. those without surgery, without adjuvant chemotherapy, etc.). Nevertheless, the hazard ratios help show either the reduced risk or increased risk of an event for the patients in these studies. Taken together, the included studies appear to demonstrate reliable performance of radiomics in accurately predicting progression-free, disease-free, and overall survival. Although prediction of survival in the adjuvant setting does not appear to be as robust as prediction of progression in the neoadjuvant setting, these data, in conjunction with other clinical, pathologic and treatment features, may help clinicians better prognosticate after surgical intervention.
3.4. Selecting patients for surgery
The use of medical imaging to accurately predict tumor behavior can significantly impact surgical decision-making and treatment planning for cancer patients. Such technology can help clinicians better identify operative candidates based on preoperative clinical and imaging variables. In hepatocelullar carcinoma, for example, identification of microvascular invasion (MVI) preoperatively may influence decisions to pursue resection or transplantation. To study the role of radiomics in predicting MVI, Zheng et al. evaluated 120 patients from two institutions who underwent resection of hepatocellular carcinoma. These data demonstrate that quantitative features could predict MVI among patients with tumors ≤5 cm with an AUC of 0.80, positive predictive value of 63 percent and negative predictive value of 85 percent [Citation55].
For patients with pancreatic intraductal papillary mucinous neoplasms (IPMN), use of imaging to predict malignant transformation is ubiquitous in clinical practice. Imaging attributes, such as cyst size, duct diameter, or presence of solid enhancing components, have been considered predictive of IPMN-associated malignancy and often mandate further evaluation with endoscopy or resection. Radiomics have been proposed as a novel method to more precisely characterize imaging features and distinguish high-risk versus low-risk IPMNs. In one retrospective series, analyzing 103 patients with IPMN, investigators sought to associate preoperative imaging features with pathologic features after resection: low-risk (low- and intermediate-grade dysplasia) versus high-risk disease (high-grade dysplasia or invasive carcinoma). A prediction model for risk assessment using clinical variables alone, including age, cyst size, presence of solid or mural nodule, symptoms and gender, was used as a comparison group. When using clinical variables alone, the multivariate prediction model demonstrated an AUC 0.67, however when combined with quantitative imaging features, the AUC was 0.79. Such data demonstrate that utilization of radiomics with clinical features may improve preoperative risk stratification and patient selection for further procedure [Citation56]. These data have been re-demonstrated and validated in later studies [Citation57,Citation58]. Such findings help clinicians appropriately select patients for upfront surgical resection. This is of particular importance for patients with precancerous lesions, such as IPMN, where timely resection may translate into a significant survival advantage.
In addition to predicting treatment response, advanced imaging techniques can improve staging accuracy prior to resection. In pancreas cancer, for example, radiomics may provide a clearer distinction between locally advanced or borderline resectable disease; such a distinction with regard to vessel involvement can assist with operative planning and prognostication. Radiomics have further been demonstrated to aid in preoperative prediction of mediastinal lymph node metastasis in lung cancer or lymph node metastasis in colorectal cancer [Citation59,Citation60]. Other studies have demonstrated the efficacy of radiomics in differentiating well-differentiated liposarcoma from lipomas or invasive lung cancers from preinvasive lesions, with significant implications for operative planning [Citation61,Citation62]. Better preoperative characterization of disease burden can improve overall outcomes by better informing surgical approach.
4. Discussion
Surgery remains a curative treatment option for many patients with solid tumors, however multimodal approaches, which combine surgery and chemotherapy or radiation, have become more prevalent with promising results [Citation63,Citation64]. Efforts to best identify patients who would benefit from surgery or multimodal approaches are increasingly critical to improve outcomes. In this paper, studies using radiomics and machine learning to predict therapeutic response, prognosis, recurrence, and survival in patients who underwent neoadjuvant or adjuvant chemotherapy were summarized to highlight the technical approaches used to generate appropriate radiomics models and a portion of the performance metrics rendered from these models. While the neoadjuvant studies are centered primarily around therapeutic response and the adjuvant studies focus on survival, both produced results that show above average performance of their models’ ability to predict outcome. Such data are promising for the future role of radiomics in clinical practice, however the feasibility of widespread expansion and adoption remains unknown.
Traditionally, clinical factors such as tumor type, stage, patient co-morbidities and functional status have influenced timing and receipt of treatment. Researchers are now focusing on analyzing and developing personalized strategies taking into account various prognostic factors [Citation65]. Radiomics has emerged as a tool to help identify treatment responders and risk-stratify patients based on projected outcomes. Radiomics coupled with robust machine learning models have shown promising results and a potential to develop personalized strategies for many cancers, including head-and-neck, breast, lung, prostate, colorectal, and gastric [Citation66]. While this summary is limited and heterogenous in tumor type, the high AUC metrics for neoadjuvant studies, ranging from 0.82 to 0.89, and adjuvant studies, ranging from 0.66 to 0.86, demonstrate the predictive power of radiomics in therapeutic response, prognosis, recurrence, and survival. These data, which suggest the reliability and reproducibility of this technique, may be clinically leveraged to guide therapy and tailor treatment to an individual patient.
4.1. Limitations and future work
Despite the promise and potential of radiomic applications, there remain many well understood challenges and limitations that need to be addressed to create robust markers for clinical use. In each of these studies, the radiomic features extracted vary greatly, with no consistency in the type of features extracted, nor the methodology used to select the final feature set [Citation67]. The imaging modalities and types of machines used to capture the medical images are vastly inconsistent at each institution [Citation67]. These differences in imaging modalities do not allow for easy replication of prior experiments. To obtain the tumor regions, radiologists need to manually segment the images. Manual segmentation is time-consuming to complete. Auto segmentation and semi-automatic segmentation methods exist but require supervision and refinement by radiologists. Radiomic studies tend to be single institution, owing to the difficulties in sharing imaging data, with no external validation of the developed models [Citation68]. The size of datasets varies, with many being too small to conclusively state that the models generated would render similar results on larger datasets. One solution to the small dataset issue is to combine datasets from multiple institutions [Citation69]. However, many medical, industrial, and international politics prevent these large datasets from being readily formed.
For radiomic studies to advance past preliminary results, an adherence and standardization of common practices has to occur [Citation67]. Multicenter data sharing and data scalability would allow for deeper studies and more robust results [Citation69]. Federated learning techniques are one possible solution to safe and secure multicenter data sharing. Federated learning seeks to address the problem of privacy and data governance by collaboratively training algorithms without exchanging the data itself [Citation70]. Privatization methods are needed to secure data and encrypt it from attackers and strengthen the appeal of federated learning as a trusted method of multicenter data sharing [Citation71]. The development of accurate, simple-to-use auto segmentation algorithms would enable radiologists to analyze more data. Implementing multimodal data approaches integrating quantitative, clinical, histological, genomic, and radiomic data is essential in designing personalized treatments [Citation68].
Recently, there have been efforts proposed to standardize radiomic features and develop strong practices that the medical machine learning community could adopt as a standard. The Image Biomarker Standardization Initiative (IBSI) is a collaboration between 25 research groups who validated consensus-based reference values for 169 radiomics features [Citation72]. The results of this study showed the potential to enable calibration and verification of radiomics software, if widely accepted as a standard.
Additionally, transparency in the construction and implementation of prediction models is proposed as a solution to curtail the lack of detail given in many papers regarding the models. The Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) Initiative was developed during a series of meetings between methodologists, health care professionals, and journal editors as a set of recommendations for the reporting of studies developing, validating, or updating a prediction model, whether for diagnostic or prognostic purposes [Citation73].
5. Conclusion
Radiomics have shown great promise in personalizing chemotherapy treatment and options for patients. The studies analyzed in this paper have demonstrated the effectiveness of radiomics and machine learning to discern the patients that are positively affected by chemotherapy versus those in whom it is futile. The standardization of radiomic analysis, increased data sharing, and stronger auto-segmentation algorithms are needed to improve the adoption and implementation in clinical applications.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organization. Fact sheet: cancer. Geneva, Switzerland: WHO; 2018.
- Schirrmacher V. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment. Int J Oncol. 2019;54(2):407–419.
- Arruebo M, Vilaboa N, Saez-Gutierrez B, et al. Assessment of the evolution of cancer treatment therapies. Cancers. 2011;3(3):3279–3330.
- Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. 2017;35(5):515–522.
- Njei B. Surgery vs. chemo-radiotherapy plus surgery in resectable esophageal cancer: a systematic review of randomized controlled trials. Gastrointest Cancer Res. 2012;5(4 Suppl 2):S9.
- Liu SV, Melstrom L, Yao K, et al. Neoadjuvant therapy for breast cancer. J Surg Oncol. 2010;101(4):283–291.
- Julien LA, Thorson AG. Current neoadjuvant strategies in rectal cancer. J Surg Oncol. 2010;101(4):321–326.
- Fathi AT, Brahmer JR. Chemotherapy for advanced stage non-small cell lung cancer. Semin Thorac Cardiovasc Surg. 2008;20(3):210–216.
- Balmanoukian A, Ettinger DS. Managing the patient with borderline resectable lung cancer. Oncology. 2010;24(3):234–241.
- Hayes DF, Schott AF. Neoadjuvant chemotherapy: What are the benefits for the patient and for the investigator? J Natl Cancer Inst Monogr. 2015;2015(51):36–39.
- Glynne-Jones R, Grainger J, Harrison M, et al. Neoadjuvant chemotherapy prior to preoperative chemoradiation or radiation in rectal cancer: Should we be more cautious? Br J Cancer. 2006;94(3):363–371.
- Browner I, Purtell M. Chemotherapy in the older patient with operable non-small cell lung cancer: neoadjuvant and adjuvant regimens. Thorac Surg Clin. 2009;19(3):377–389.
- Rosenbaum E. Everyone’s guide to cancer supportive care: a comprehensive handbook for patients and their families. Kansas City (MO): Andrews McMeel Publishing; 2005.
- DeVita VT, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68(21):8643–8653.
- Sevin B-U, Knapstein PG, Kochli OR, et al. Multimodality therapy in gynecologic oncology. New York (NY): Thieme Publishing Group; 1996.
- Tao JJ, Visvanathan K, Wolff AC. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. The Breast. 2015;24:S149–S153.
- Baker J, Kornguth PJ, Soo MS, et al. Sonography of solid breast lesions: observer variability of lesion description and assessment. AJR Am J Roentgenol. 1999;172(6):1621–1625.
- Van de Steene J, Linthout N, de Mey J, et al. Definition of gross tumor volume in lung cancer: Inter-observer variability. Radiother Oncol. 2002;62(1):37–49.
- Yip SS, Liu Y, Parmar C, et al. Associations between radiologist-defined semantic and automatically computed radiomic features in non-small cell lung cancer. Sci Rep. 2017;7(1):1–11.
- Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48(4):441–446.
- Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images are more than pictures, they are data. Radiology. 2016;278(2):563–577.
- Parekh V, Jacobs MA. Radiomics: a new application from established techniques. Expert Rev Precis Med Drug Dev. 2016;1(2):207–226.
- Biomarkers in cancer: introductory guide for advocates. Research Advocacy Network. 2010.
- Wei W, Liu Z, Rong Y, et al. A computed tomography-based radiomic prognostic marker of advanced high-grade serous ovarian cancer recurrence: a multicenter study. Front Oncol. 2019;9:255.
- Shu Z, Fang S, Ding Z, et al. MRI-based radiomics nomogram to detect primary rectal cancer with synchronous liver metastases. Sci Rep. 2019;9(1):1–10.
- Jiang Y, Chen C, Xie J, et al. Radiomics signature of computed tomography imaging for prediction of survival and chemotherapeutic benefits in gastric cancer. EBioMedicine. 2018;36:171–182.
- Davis AM, Bell RS, Goodwin PJ. Prognostic factors in osteosarcoma: a critical review. J Clin Oncol. 1994;12(2):423–431.
- Lin P, Yang P-F, Chen S, et al. A Delta-radiomics model for preoperative evaluation of neoadjuvant chemotherapy response in high-grade osteosarcoma. Cancer Imag. 2020;20(1):7.
- Drukker K, Edwards AV, Doyle C, et al. Breast MRI radiomics for the pretreatment prediction of response to neoadjuvant chemotherapy in node-positive breast cancer patients. J Med Imaging. 2019;6(3):034502.
- Braman N, Prasanna P, Whitney J, et al. Association of peritumoral radiomics with tumor biology and pathologic response to preoperative targeted therapy for HER2 (ERBB2)– positive breast cancer. JAMA Netw Open. 2019;2(4):e192561–e192561.
- Li Z, Zhang D, Dai Y, Department of Radiology, the Third Affiliated Hospital of Kunming Medical University, Yunnan Cancer Hospital, Kunming 650118, China, et al. Computed tomography-based radiomics for prediction of neoadjuvant chemotherapy outcomes in locally advanced gastric cancer: a pilot study. Chin J Cancer Res. 2018;30(4):406–414.
- Zhou J, Lu J, Gao C, et al. Predicting the response to neoadjuvant chemotherapy for breast cancer: Wavelet transforming radiomics in MRI. BMC Cancer. 2020;20(1):100.
- Khalvati F, Zhang Y, Baig S, et al. Prognostic value of CT radiomic features in resectable pancreatic ductal adenocarcinoma. Sci Rep. 2019;9(1):1–9.
- Wang J, Shen L, Zhong H, et al. Radiomics features on radiotherapy treatment planning CT can predict patient survival in locally advanced rectal cancer patients. Sci Rep. 2019;9(1):1–9.
- Wang Q, Zhou S, Court LE, et al. Radiomics predicts clinical outcome in primary gastroesophageal junction adenocarcinoma treated by chemo/radiotherapy and surgery. Phys Imag Radiat Oncol. 2017;3:37–42.
- Rabinovici-Cohen S, Tlusty T, Abutbul A, et al. Radiomics for predicting response to neoadjuvant chemotherapy treatment in breast cancer. In: Medical imaging 2020: imaging informatics for healthcare, research, and applications. Vol. 113181B. Houston (TX): International Society for Optics and Photonics; 2020.
- Tian X, Sun C, Liu Z, et al. Prediction of response to preoperative neoadjuvant chemotherapy in locally advanced cervical cancer using multicenter CT-based radiomic analysis. Front Oncol. 2020;10:77.
- Liu Z, Li Z, Qu J, et al. Radiomics of multiparametric MRI for pretreatment prediction of pathologic complete response to neoadjuvant chemotherapy in breast cancer: a multicenter study. Clin Cancer Res. 2019;25(12):3538–3547.
- Jiang Y, Wang H, Wu J, et al. Noninvasive imaging evaluation of tumor immune microenvironment to predict outcomes in gastric cancer. Ann Oncol. 2020;31(6):760–768.
- Bourbonne V, Fournier G, Vallieres M, et al. External validation of an MRI-derived radiomics model to predict biochemical recurrence after surgery for high-risk prostate cancer. Cancers. 2020;12(4):814.
- Pavic M, Bogowicz M, Kraft J, et al. FDG PET versus CT radiomics to predict outcome in malignant pleural mesothelioma patients. EJNMMI Res. 2020;10(1):1–8.
- Spraker MB, Wootton LS, Hippe DS, et al. MRI radiomic features are independently associated with overall survival in soft tissue sarcoma. Adv Radiat Oncol. 2019;4(2):413–421.
- Bani-Sadr A, Eker OF, Berner L-P, et al. Conventional MRI radiomics in patients with suspected early- or pseudo-progression . Neurooncol Adv. 2019;1(1):vdz01.
- Wormald BW, Doran SJ, Ind TE, et al. Radiomic features of cervical cancer on T2-and diffusion-weighted MRI: prognostic value in low-volume tumors suitable for trachelectomy. Gynecologic Oncology. 2020;156(1):107–114.
- Xie D, Wang T-T, Huang S-J, et al. Radiomics nomogram for prediction disease-free survival and adjuvant chemotherapy benefits in patients with resected stage I lung adenocarcinoma. Transl Lung Cancer Res. 2020;9(4):1112–1123.
- Vaidya P, Bera K, Gupta A, et al. CT derived radiomic score for predicting the added benefit of adjuvant chemotherapy following surgery in stage I, II resectable non-small cell lung cancer: a retrospective multicohort study for outcome prediction. The Lancet Digit Health. 2020;2(3):e116–e128.
- Deng L, Yu D. Deep learning: Methods and applications. FNT Sig Process. 2014;7(3–4):197–387.
- Kaissis G, Braren R. Pancreatic cancer detection and characterization—state of the art cross-sectional imaging and imaging data analysis. Transl Gastroenterol Hepatol. 2019;4:35–35.
- Becker AS, Schneider MA, Wurnig MC, et al. Radiomics of liver MRI predict metastases in mice. Eur Radiol Exp. 2018;2(1):10–11.
- Sun K-Y, Hu H-T, Chen S-L, et al. CT-based radiomics scores predict response to neoadjuvant chemotherapy and survival in patients with gastric cancer. BMC Cancer. 2020;20(1):1–11.
- Conroy T, Lamfichekh N, Etienne P-L, et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. 2020;4007.
- Liu Z, Zhang X-Y, Shi Y-J, et al. Radiomics analysis for evaluation of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Clin Cancer Res. 2017;23(23):7253–7262.
- Coroller TP, Agrawal V, Narayan V, et al. Radiomic phenotype features predict pathological response in non-small cell lung cancer. Radiother Oncol. 2016;119(3):480–486.
- Braman NM, Etesami M, Prasanna P, et al. Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res. 2017;19(1):1–14.
- Zheng J, Chakraborty J, Chapman WC, Research Staff in the Department of Surgery at Washington University School of Medicine, et al. Preoperative prediction of microvascular invasion in hepatocellular carcinoma using quantitative image analysis. J Am Coll Surg. 2017;225(6):778–788.
- Attiyeh MA, Chakraborty J, Gazit L, et al. Preoperative risk prediction for intraductal papillary mucinous neoplasms by quantitative CT image analysis. HPB. 2019;21(2):212–218.
- Polk SL, Choi JW, McGettigan MJ, et al. Multiphase computed tomography radiomics of pancreatic intraductal papillary mucinous neoplasms to predict malignancy. WJG. 2020;26(24):3458–3471.
- Tobaly D, Santinha J, Sartoris R, et al. Ct-based radiomics analysis to predict malignancy in patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Cancers. 2020;12(11):3089.
- Zhong Y, Yuan M, Zhang T, et al. Radiomics approach to prediction of occult mediastinal lymph node metastasis of lung adenocarcinoma. AJR Am J Roentgenol. 2018;211(1):109–113.
- Huang Y-Q, Liang C-H, He L, et al. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J Clin Oncol. 2016;34(18):2157–2164.
- Vos M, Starmans M, Timbergen M, et al. Radiomics approach to distinguish between well differentiated liposarcomas and lipomas on MRI. Br J Surg. 2019;106(13):1800–1809.
- Wu G, Woodruff HC, Sanduleanu S, et al. Preoperative CT-based radiomics combined with intraoperative frozen section is predictive of invasive adenocarcinoma in pulmonary nodules: a multicenter study. Eur Radiol. 2020;30(5):2612–2680.
- Van Tienhoven G, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC1): a randomized, controlled, multicenter phase III trial. ASCO. 2018;LBA4002-LBA4002.
- Yang H, Liu H, Chen Y, on behalf of the AME Thoracic Surgery Collaborative Group, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36(27):2796–2803.
- Overdevest JB, Theodorescu D, Lee JK. Utilizing the molecular gateway: the path to personalized cancer management. Clin Chem. 2009;55(4):684–697.
- Liu Z, Wang S, Di Dong JW, et al. The applications of radiomics in precision diagnosis and treatment of oncology: Opportunities and challenges. Theranostics. 2019;9(5):1303–1322.
- Lambin P, Leijenaar RT, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–762.
- Crivelli P, Ledda RE, Parascandolo N, et al. A new challenge for radiologists: radiomics in breast cancer. Biomed Res Int. 2018;2018:6120703.
- Qiu Q, Duan J, Yin Y. Radiomics in radiotherapy: applications and future challenges. Prec Radiat Oncol. 2020;4(1):29–33.
- Rieke N, Hancox J, Li W, et al. The future of digital health with federated learning. NPJ Digit Med. 2020;3(1):1–7.
- Kaissis GA, Makowski MR, Ruckert D, et al. Secure, privacy-preserving and federated machine learning in medical imaging. Nat Mach Intell. 2020;2(6):305–311.
- Zwanenburg A, Vallieres M, Abdalah MA, et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. 2020;295(2):328–338.
- Collins GS, Reitsma JB, Altman DG, TRIPOD Group, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. The TRIPOD Group. Circulation. 2015;131(2):211–219.