Abstract
Purpose
To explore the effect of image-guided systems in phacoemulsification with intraocular lens (IOL) implantation.
Methods
We searched Pubmed, Embase and China National Knowledge Infrastructure (inception to January 20, 2021). Two researchers extracted data and assessed paper quality independently. Uncorrected distance visual acuity (UDVA) before and after surgery, best corrected visual acuity (BCVA) before and after surgery, preoperative cylinder, postoperative residual refractive cylinder, postoperative corneal cylinder, IOL misalignment, and intraocular pressure (IOP) were compared.
Results
We included 14 studies with 885 cataract eyes. All data were performed using Review Manager 5.3 (RevMan 5.3) (https://revman.cochrane.org/). Cases of all preoperative outcomes showed no significant difference between image-guided group and manual group. There was no significant difference in postoperative UDVA (Standard mean difference (SMD: −0.11, 95% CI: −0.32 to 0.11, I2 = 59%, p = 0.33)), BCVA (SMD: 0.03, 95% CI: −0.12 to 0.18, I2 = 36%, p = 0.72), corneal cylinder (Weighted mean difference WMD: 0.13, 95% CI: −0.06 to −0.32, I2 = 0%, p = 0.17), IOP (WMD: −0.37, 95% CI: −1.36 to −0.62, I2 = 9%, p = 0.46) between two groups. There was less residual refractive cylinder in image-guided group than in manual group (WMD: −0.20, 95% CI: −0.26 to −0.14, I2 = 59%, p<0.00001). It is more accurate in IOL alignment when combined with image-guided systems (WMD: −1.20, 95% CI: −1.43 to −0.96, I2 = 14%, p < 0.00001).
Conclusion
Image-guided systems can improve the effect in phacoemulsification with intraocular lens (IOL) implantation.
Introduction
Cataract is a group of opacity in the lens as a common feature of the disease, one of the major blinding eye diseases in the world, and surgery is the main treatment. 34.7%–47.3% of cataract patients had corneal cylinder≥ 1.0 D, which seriously affected the visual function after refractive surgery [Citation1]. Intraocular lens (IOL) implantation is a safe and effective modality in such cases to help treat cataract at the same time of correcting regular cylinder of cornea [Citation2,Citation3]. Accurate measurement of preoperative corneal cylinder, IOL axis alignment along the desired corneal meridian and intraoperative positioning are necessary. One degree of misalignment results in 3.3% decrease in the correction of cylinder, and 30° misalignment effectively increase the amount of preoperative cylinder [Citation4–6].
Manual marking has been widely used, such as bubble, direct vision in the slitlamp, ink, pendulum and tonometer [Citation7]. With the development of digitization, imaged-guided systems are gradually used in cataract surgery, such as the Callisto Eye with ZAlign (Carl Zeiss Meditec AG, Jena, Germany), the TrueGuide software (TrueVision 3 D Surgical, Inc., Santa Barbara, CA), and the VERION Digital Marker (Alcon Laboratories, Ft. Worth, TX) [Citation8–10]. Compared with manual marking, the image-guided systems have the functions of intraoperative projection, incision positioning, assisted capsulorhexis trajectory and toric IOL positioning.
However, which method is the better remains controversial. Three studies [Citation11–13] suggested that image-guided systems have a significant effect on improving the vision acuity in phacoemulsification with IOL implantation. Five studies [Citation12–16] suggested that there were less residual refractive cylinder in image-guided marking group than in manual marking group while other four studies [Citation17–20] didn’t. In addition to IOL misalignment, strong evidence indicates that it was more accurate when combined with image-guided systems [Citation13,Citation15–22]. And two studies [Citation14,Citation23] suggested accurate manual marking and digital marking were equally effective guides for toric IOL alignment intraoperatively.
The aim of this systematic review and meta-analysis is to identify, evaluate and summarize the available evidence to determine if image-guided systems have an overall significant improvement in phacoemulsification with IOL implantation.
Material and methods
Literature search
The meta-analysis was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The Pubmed, Embase and China National Knowledge Infrastructure (inception to January 20, 2021) databases were searched for cohort studies. The search terms were “(cataract OR IOL) AND (digital OR guided OR verion)” and they were applied without language restrictions. Studies from other sources were added if they met the inclusion criteria.
Study eligibility and selection
The following studies were included:
Participants’ eyes with corneal cylinder were at least 1.00 D,
Participants’ eyes had cataract surgery and toric IOL implantation,
Surgery used both image-guided and manual marking techniques for toric alignment.
Studies were excluded if
Patients were with ocular pathology such as preexisting glaucoma, retinal pathology, small pupils, corneal opacity, ocular surface disorders, or systemsic conditions, or medication use expected to result in a postoperative visual acuity worse than 20/30,
Patients currently used contact lenses or had previous refractive surgery or conditions with the potential to result in a complicated cataract surgery,
Eyes were with intraoperative complications such as extension of capsulorhexis, posterior capsular rent, vitreous loss, and nucleus drop.
Each study was initially screened by two investigators (Yang Su, Guo-Guo Yi) based on their titles and abstracts, and then by reading the full text according to the inclusion and exclusion criteria, with any disagreement resolved by consultation with a third investigator (Min Fu).
Quality assessment
The quality of the papers was evaluated using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool (http://www.bristol.ac.uk/population-health-sciences/projects/quadas/) from the following 4 aspects: patient selection, index text, reference standard, flow and timing.
Data extraction
We extracted the following data from each study:
Study characteristics, including the first author, publication year, study design, surgery method, number of patients, age,
Outcome measures, including uncorrected distance visual acuity (UDVA) before and after surgery, best corrected visual acuity(BCVA) before and after surgery, preoperative cylinder, postoperative residual refractive cylinder, postoperative corneal cylinder, IOL misalignment, and intraocular pressure(IOP).
Data synthesis and statistical analysis
All measures we extracted (UDVA, BCVA, cylinder, IOL misalignment, IOP) were reported as the mean ± SD. UDVA and BCVA before and after surgery were reported by decimal counting in the two studies [Citation12,Citation13] and log MAR in other studies, therefore analyzed by the standard mean difference (SMD). Cylinder, IOL misalignment and IOP were analyzed by weighted mean difference (WMD). Subgroup analyses according to the follow-up time were performed by grouping studies that used the same tool. p < 0.05 was considered statistically significant.
The I2 statistic was assessed to measure the degree of inconsistency and quantify the effect of heterogeneity in the results of the trials. Outcomes from the selected trials were combined with a fixed-effect model if I2 was less than 50%. The random-effect model was applied if I2 was greater than 50%, which indicates substantial heterogeneity. Sensitivity analyses were performed first by removing the studies with the highest risk of bias and then by removing one study at a time to verify whether the results would change. All statistical analyses were conducted using Review Manager Software (Version 5.3, The Nordic Cochrane Center, The Cochrane Collaboration, 2014, Copenhagen) (https://revman.cochrane.org/).
Results
Characteristics of studies
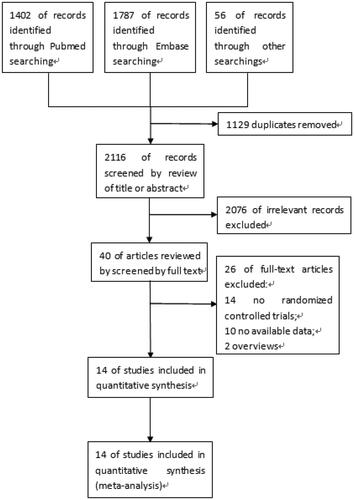
A total of 3245 articles were initially identified, with 1129 of these duplicates, leaving 2116. Following the screening of titles and abstracts, 40 articles were retrieved for full-text review. 14 articles [Citation11–24] that met the inclusion criteria were finally included. The flowchart () showed the literature search process.
The characteristics of the included studies are showed in . The studies were published between 2014 and 2020 and included a total number of 885 eyes (469 in image-guided group and 416 in manual group) in phacoemulsification with IOL implantation. The duration of the follow up ranged from 1 day to 3 months.
Table 1. Characteristics of included studies.
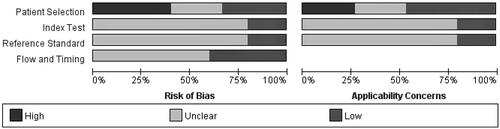
and showed the risk of bias and applicability concerns in all. Four studies [Citation15,Citation16,Citation22,Citation23] did not include information on whether they enrolled a consecutive series of patients and were case control studies, resulting in high degrees of risk for bias in patient selection. How and who measured refraction has the potential to bias the study endpoint. Few studies mention whether the index test results were interpreted without knowledge of the results of the reference standard and whether the reference standard results were interpreted without knowledge of the results of the index test.
Table 2. Quality assessment.
Preoperative outcomes
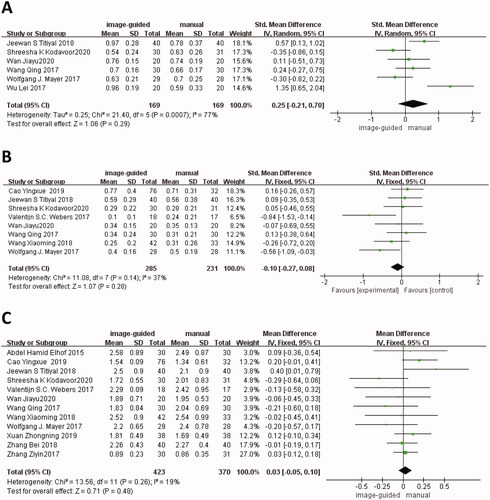
There were six studies [Citation12–16,Citation21] reported preoperative UDVA, eight studies [Citation11–15,Citation18,Citation21,Citation22] reported preoperative BCVA, and twelve studies [Citation11–15,Citation17–22,Citation24] reported preoperative cylinder. There was no significant difference in UDVA (SMD: 0.25, 95% CI: −0.21 to 0.70, I2 = 77%, p = 0.29), BCVA (SMD: −0.10, 95% CI: −0.27 to 0.08, I2 = 37%, p = 0.28) and cylinder (WMD: 0.03, 95% CI: −0.05 to 0.10, I2 = 19%, p = 0.48) between image-guided group and manual group ().
Postoperative outcomes
UDVA
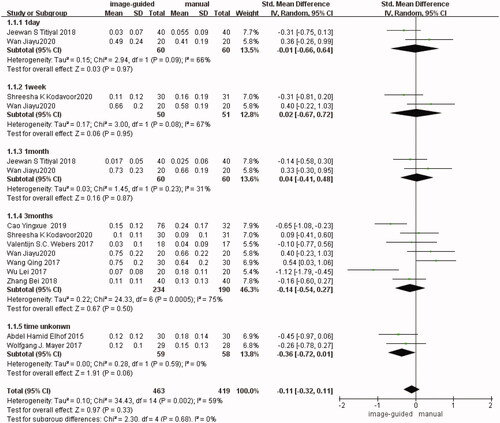
It is divided into five subgroups according to the follow-up time in UDVA. There were two studies [Citation12,Citation15] reported in the first subgroup (1 day after surgery) (SMD: −0.01, 95% CI: −0.66 to 0.64, I2 = 66%, p = 0.97), two studies [Citation14,Citation15] in the second subgroup (1 week after surgery) (SMD: 0.02, 95% CI: −0.67 to 0.72, I2 = 67%, p = 0.95), two studies [Citation12,Citation15] in the third subgroup (1 month after surgery) (SMD: 0.04, 95% CI: −0.41 to 0.48, I2 = 31%, p = 0.87), seven studies [Citation11–14,Citation16,Citation18,Citation20] in the fourth subgroup (3 months after surgery) (SMD: −0.14, 95% CI: −0.54 to 0.27, I2 = 75%, p = 0.50), and two studies [Citation17,Citation21] in the fifth subgroup (the follow-up time unknown) (SMD: −0.36, 95% CI: −0.72 to 0.01, I2 = 0%, p = 0.06). There was no significant difference in each subgroup respectively and in the total group (SMD: −0.11, 95% CI: −0.32 to 0.11, I2 = 59%, p = 0.33) between image-guided group and manual group ()
BCVA
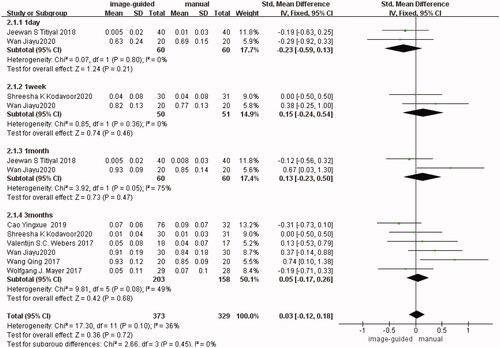
It is divided into four subgroups according to the follow-up time in BCVA. There were two studies [Citation12,Citation15] reported in the first subgroup (1 day after surgery) (SMD: −0.23, 95% CI: −0.59 to 0.13, I2 = 0%, p = 0.21), two studies [Citation12,Citation14] in the second subgroup (1 week after surgery) (SMD: 0.15, 95% CI: −0.24 to 0.54, I2 = 0%, p = 0.46), two studies [Citation12,Citation15] in the third subgroup (1 month after surgery) (SMD: 0.24, 95% CI: −0.52 to 1.00, I2 = 74%, p = 0.54), six studies [Citation11–14,Citation18,Citation21] in the fourth subgroup (3 months after surgery) (SMD: 0.05, 95% CI: −0.17 to 0.26, I2 = 49%, p = 0.68). There was no significant difference in each subgroup respectively and in the total group (SMD: 0.03, 95% CI: −0.12 to 0.18, I2 = 36%, p = 0.72) between image-guided group and manual group ().
Residual refractive cylinder
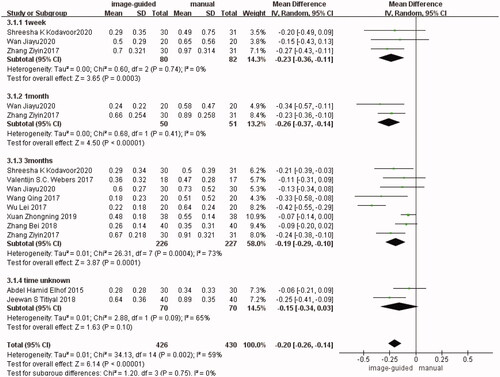
It was divided into four subgroups according to the follow-up time. There were three studies [Citation12,Citation14,Citation24] reported in the first subgroup (1 week after surgery) (WMD: −0.23, 95% CI: −0.36 to −0.11, I2 = 0%, p = 0.0003), two studies [Citation12,Citation24] in the second subgroup (1 month after surgery) (WMD: −0.26, 95% CI: −0.37 to −0.14, I2 = 0%, p < 0.00001), eight studies [Citation12–14,Citation16,Citation18–20,Citation24] in the third subgroup (3 months after surgery) (WMD: −0.19, 95% CI: −0.29 to −0.10, I2 = 73%, p = 0.0001), and two studies [Citation15,Citation17] in the fourth subgroup (the follow-up time unknown) (WMD: −0.15, 95% CI: −0.34 to 0.03, I2 = 65%, p = 0.10). Although the difference was not statistically significant in the fourth group, it was statistically significant in other three subgroups respectively and in the total group (WMD: −0.20, 95% CI: −0.26 to −0.14, I2 = 59%, p < 0.00001) between image-guided group and manual group ().
Corneal cylinder
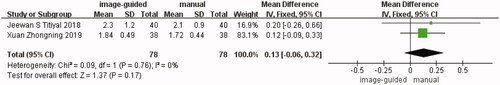
Two studies [Citation15,Citation19] showed a comparison of corneal cylinder between the image-guided group and manual group. The difference was not statistically significant (WMD: 0.13, 95% CI: −0.06 to −0.32, I2 = 0%, p = 0.17) ().
IOL misalignment
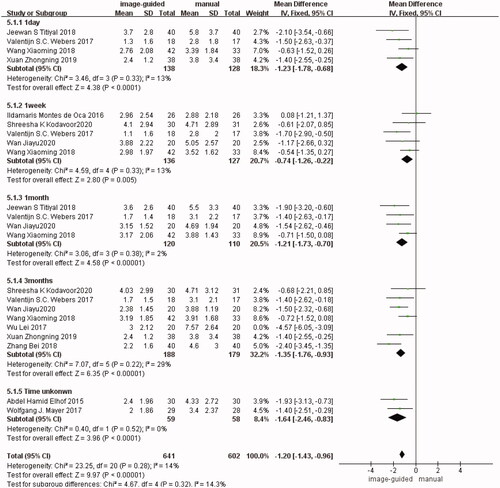
It is divided into five subgroups according to the follow-up time. There were four studies [Citation15,Citation18,Citation19,Citation22] reported in the first subgroup (1 day after surgery) (WMD: −1.23, 95% CI: −1.78 to −0.68, I2 = 13%, p < 0.0001), five studies [Citation12,Citation14,Citation18,Citation22,Citation23] in the second subgroup (1 week after surgery) (WMD: −0.74, 95% CI: −1.26 to −0.22, I2 = 13%, p = 0.005), four studies [Citation12,Citation15,Citation18,Citation22] in the third subgroup (1 month after surgery) (WMD: −1.21, 95% CI: −1.73 to −0.70, I2 = 2%, p < 0.00001), seven studies [Citation12,Citation14,Citation16,Citation18–20,Citation22] in the fourth subgroup (3 months after surgery) (WMD: −1.35, 95% CI: −1.76 to −0.93, I2 = 29%, p< 0.00001), two studies [Citation17,Citation21] in the fifth subgroup (the follow-up time unknown) (WMD: −1.64, 95% CI: −2.46 to −0.83, I2 = 0%, p< 0.0001). There was no significant difference in each subgroup respectively and in the total group (WMD: −1.20, 95% CI: −1.43 to −0.96, I2 = 14%, p<0.00001) between image-guided group and manual group ().
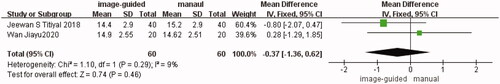
IOP
Two studies [Citation12,Citation15] showed a comparison of IOP between the image-guided group and manual group. The difference was not statistically significant (WMD: −0.37, 95% CI: −1.36 to −0.62, I2 = 9%, p = 0.46) ().
Heterogeneity analysis
Postoperative UDVA (I2 = 59%) and residual refractive cylinder (I2 = 59%) have high heterogeneity. After excluding Cao and Wu’s studies [Citation11,Citation16] in UDVA, the heterogeneity significantly decreased in the fourth group (3 months after surgery) (SMD: 0.13, 95% CI: −0.11 to 0.37, I2 = 26%, p = 0.27) and in the total group (SMD: −0.02, 95% CI: −0.17 to 0.12, I2 = 34%, p = 0.76). The difference was still not statistically significant. After excluding Wu’s study [Citation16], the heterogeneity significantly decreased in the fourth group (3 months after surgery) (WMD: −0.13, 95% CI: −0.17 to −0.08, I2 = 33%, p<0.00001) and in the total group (WMD: −0.15, 95% CI: −0.19 to −0.11, I2 = 33%, p<0.00001), the result did not change and it was still statistically significant. Other outcomes all have low heterogeneity.
Discussion
With the development of digital technology, cataract patients have high expectations for the curative effect of surgery. Two main factors are essential: the insertion of a correct power IOL due to accurate keratometry [Citation25,Citation26] and accurate alignment of toric IOL. Image-guided systems can not only provide keratometry (K) measurements, but provide digital image guidance for toric IOL alignment without preoperative manual marking. Image-guided systems were designed to help the surgeon with a preoperative planning of the size and location of the surgical incisions and capsulorrhexis, as well as correct placement of IOLs and accurate alignment of toric IOLs. These systems give the opportunity to the surgeon to see the aforementioned measurements intraoperatively in real time [Citation26,Citation27].
This meta-analysis is to explore the effect of image-guided systems in phacoemulsification with IOL implantation. The difference was not statistically significant in all preoperative measurements we extracted (UDVA, CDVA and cylinder), making our postoperative results more accurate credible. 3 methods (Verion, Alcon Laboratories, Inc.; Callisto Eye Systems, Carl Zeiss Meditec AG; and TrueVision 3 D, TrueVision Systems, Inc.) were included in the image-guided group and 4 methods (bubble, pendulum, direct visualization by the silmlamp and ink) in the manual group, increasing the heterogeneity. And we divided postoperative outcomes (UDVA, CDVA, residual refractive cylinder and IOL misalignment) into different subgroups according to the following-up time ranging from 1 day to 3 months, decreasing the heterogeneity [Citation28,Citation29]. In the analyses above, residual refractive cylinder had high heterogeneity. After excluding the study [Citation16] of high heterogeneity, the result did not change and it was still statistically significant, which mean our results were credible.
The results of this meta-analysis suggest that image-guided systems can improve the effect in cataract surgery. Combined with image-guided systems, less residual refractive cylinder and more accurate IOL alignment were in phacoemulsification with IOL implantation. Although no significantly difference in postoperative UDVA and BCVA, the vision acuity in image-guided groups were commonly better than in manual groups. Larger-sample experiments should be performed to verify if image-guided systems can really have a significant effect on improving vision acuity. It was not statistically significant in postoperative UDVA, BCVA, corneal cylinder and IOP, suggesting that manual marking can also achieve high surgical efficacy in the condition of limited economic.
Also, there were some limitations in this meta-analysis: (1) the sample size we included was small, and a large number of studies were still needed; (2) the digital systems in image-guided groups (Verion, Alcon Laboratories, Inc.; Callisto Eye Systems, Carl Zeiss Meditec AG; and TrueVision 3 D, TrueVision Systems, Inc.) and the manual marking methods (bubble, pendulum, direct visualization by the silmlamp and ink) in manual groups were different; (3) most of the included studies did not adopt blind method and allocation concealment, and there was the possibility of selective bias, implementation bias and measurement bias; (4) the number of some indicators included (postoperative corneal cylinder and IOP) was small, and the statistical efficiency may be insufficient. In conclusion, image-guided systems can improve the effect in phacoemulsification with intraocular lens (IOL) implantation. And more high-quality and large-scale clinical studies are needed.
Ethics statement
This article is based on previously published studies and does not contain any studies with human participants performed by any of the authors.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure statement
Yang Su, Min Fu, Yifan Chen, Rui Qiao and Guo-Guo Yi declare no personal, financial, commercial, or academic conflicts of interest, and none of the authors have a financial or proprietary interest in a product, method, or material in this paper.
Data availability statement
The datasets generated during and/or analyses during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Kim H, Whang WJ, Joo CK. Corneal astigmatism in patients after cataract surgery: a 10-Year follow-up study. J Refract Surg. 2016;32(6):404–409.
- Collier Wakefield O, Annoh R, Nanavaty MA. Relationship between age, corneal astigmatism, and ocular dimensions with reference to astigmatism in eyes undergoing routine cataract surgery. Eye. 2016;30(4):562–569.
- Hoffmann PC, Hütz WW. Analysis of biometry and prevalence data for corneal astigmatism in 23,239 eyes. J Cataract Refract Surg. 2010;36(9):1479–1485.
- Cha D, Kang SY, Kim S-H, et al. New axis-marking method for a toric intraocular lens: mapping method. J Refract Surg. 2011;27(5):375–379.
- Tognetto D, Perrotta AA, Bauci F, et al. Quality of images with toric intraocular lenses. J Cataract Refract Surg. 2018;44(3):376–381.
- Visser N, Berendschot TT, Bauer NJ, et al. Accuracy of toric intraocular lens implantation in cataract and refractive surgery. J Cataract Refract Surg. 2011;37(8):1394–1402.
- Nina P, Nino H, Sophie M, et al. Evaluation of 4 corneal astigmatic marking methods. J Cataract Refract Surg. 2012;38(12):2094–2099.
- Brooks CC, Kitchens J, Stone TW, et al. Consolidation of imaging modalities utilizing digitally assisted visualization systems: the development of a surgical information handling cockpit. Clin Ophthalmol. 2020;14:557–569.
- Dubinsky-Pertzov B, Hecht I, Gazit I, et al. Clinical outcomes of ankoris toric intraocular lens implantation using a computer-assisted marker system. Int Ophthalmol. 2020;40(12):3259–3267.
- Ventura BV, Wang L, Weikert MP, et al. Surgical management of astigmatism with toric intraocular lenses. Arq Bras Oftalmol. 2014;77(2):125–131.
- Cao YX, Wang J. Incipient observation of cornea astigmatism after combined cataract phacoemulsification and callisto eye assisted arcuate keratotomy. Chin J Ophthalmol. 2019;55(7):502–508.
- Wan JY. Effect of different toric intraocular lens marking methods on postoperative visual effects. North China University of Science and Technolegy.
- Wang Q. Clinical application of VERION image guided system in toric IOL implantation. TIANJIN Medical University. 2017.
- Kodavoor SK, Divya J, Dandapani R, et al. Randomized trial comparing visual outcomes of toric intraocular lens implantation using manual and digital marker. Indian J Ophthalmol. 2020;68(12):3020–3024.
- Titiyal JS, Kaur M, Jose CP, et al. Comparative evaluation of toric intraocular lens alignment and visual quality with image-guided surgery and conventional three-step manual marking. OPTH. 2018;12:747–753.
- Wu L, Wang WH. The application of verion system in toric intraocular lens implantation. Chin J Ocul Traum Occupat Eye Dis. 2017;39(9):641–643.
- Elhofi AH, Helaly HA. Comparison between digital and manual marking for toric intraocular lenses: a randomized trial. Medicine. 2015;94(38):e1618.
- Webers VSC, Bauer NJC, Visser N, et al. Image-guided system versus manual marking for toric intraocular lens alignment in cataract surgery. J Cataract Refract Surg. 2017;43(6):781–788.
- Xuan XN. Application of VERION digital navigation system to intraocular lens implantation for astigmatism. Guangxi Med J. 2019;41(8):954–957.
- Zhang B, Deng GH, Zhou D, et al. Comparison between verion digital and manual marking for toric intraocular lenses. Chin J Optom Ophthalmol. 2018;20(4):227–231.
- Mayer WJ, Thomas K, Martin D, et al. Comparison of visual outcomes, alignment accuracy, and surgical time between 2 methods of corneal marking for toric intraocular lens implantation. J Cataract Refract Surg. 2017;43(10):1281–1286.
- Wang XM, Tang X, Zheng XH. Comparison of toric intraocular lens axis marking using VERION image guided system and slit lamp observation. Rec Adv Ophthalmol. 2018;38(2):153–160.
- Montes de Oca I, Kim E, Wang L, et al. Accuracy of toric intraocular lens axis alignment using a 3-dimensional computer-guided visualization system. J Cataract Refract Surg. 2016;42(4):550–555.
- Zhang ZY. Intraoperative navigation system combined with postoperative 0.3% sodium hyaluronate to improve the effect of cataract surgery. NANJING Medical University. 2017.
- Brick DC. Risk management lessons from a review of 168 cataract surgery claims. Surv Ophthalmol. 1999;43(4):356–360.
- Panagiotopoulou EK, Ntonti P, Gkika M, et al. Image-guided lens extraction surgery: a systematic review. Int J Ophthalmol. 2019;12(1):135–151.
- Blaylock JF, Hall B. Astigmatic results of a diffractive trifocal toric IOL following intraoperative aberrometry guidance. OPTH. 2020;14:4373–4378.
- Hura AS, Osher RH. Comparing the Zeiss Callisto eye and the Alcon verion image guided system toric lens alignment technologies. J Refract Surg. 2017;33(7):482–487.
- Zhou FQ, Jiang W, Lin ZL, et al. Comparative meta-analysis of toric intraocular lens alignment accuracy in cataract patients: image-guided system versus manual marking. J Cataract Refract Surg. 2019;45(9):1340–1345.