Abstract
Diabetic kidney disease (DKD) is the major cause of end stage renal disease. Despite the best current treatment, it is difficult to stop the progression of DKD. We report a 77-year-old Japanese man in whom rheumatoid arthritis (RA) was diagnosed at the age of 30 years, followed by type 2 diabetes mellitus (T2DM) at age 55. His haemoglobin A1c (HbA1c) was around 7–9%, even after starting insulin therapy. His renal function deteriorated steadily, with the estimated glomerular filtration rate (eGFR) decreasing by 2 mL/min/1.73 m2 per year to reach 29 mL/min/1.73 m2 (consistent with stage IV diabetic kidney disease) at age 69. Because RA was poor controlled with a DAS28-ESR of 4.46, HAQ of 0.375, and SDAI of 8.6, treatment with tocilizumab (an interleukin-6 receptor antibody) was initiated. Thereafter, control of RA improved, but HbA1c stayed in the range of 7–9%. However, eGFR remained around 30 ml/min/1.73 m2 even after 8 years. This case indicates that interleukin-6 might contribute to exacerbation of renal dysfunction, since IL-6 receptor blockade prevented deterioration of renal function in this patient with RA and diabetic kidney disease.
Introduction
Diabetic kidney disease (DKD) is a kidney disease caused by diabetes and develops in approximately 40% of these patients. Despite the best current treatment, DKD remains one of the most frequent causes of ESRD [Citation1]. The incidence rate of diabetic end-stage renal disease (ESRD) was 15 cases/1000 person-years [Citation2]. The decline rate of estimated glomerular filtration is −0.9 mL/min/1.73 m2 in stage 4 diabetes nephropathy [Citation3]. It has been suggested that DKD is not only caused by metabolic and hemodynamic abnormalities, but also by inflammatory mediators such as tumour necrosis factor-α and interleukin (IL)-1, IL-6 and IL-18 [Citation4]. In the kidneys, IL-6 promotes the proliferation of podocytes and mesangial cells, as well as inducing endothelial cell dysfunction with vasoconstriction, resulting in glomerular sclerosis and tubular atrophy [Citation5]. Thus, an IL-6 receptor blocker might theoretically be a therapeutic option for patients with DKD, but this remains unproven.
Here, we report a 77-year-old Japanese man with T2DM and stage IV DKD accompanied by rheumatoid arthritis (RA). His renal function deteriorated steadily over a long period, but further progression was subsequently prevented for 8 years after he started treatment for RA with a humanized anti-interleukin-6 receptor antibody (tocilizumab). This case suggests a close relationship between inflammatory cytokines and the progression of DKD.
Case report
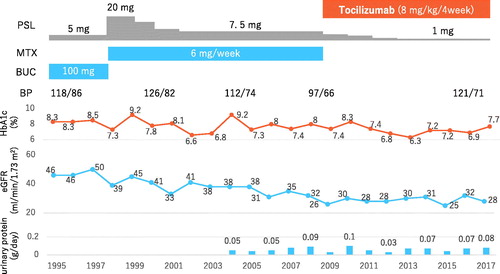
The patient was a 77-year-old Japanese man in whom RA was diagnosed at the age of 30 years. Steroids and disease-modifying antirheumatic drugs, including bucillamine, were administered. Twenty-five years after the initiation of steroid treatment, he was diagnosed with T2DM when he was aged 55 years. Even after insulin was started, HbA1c was poorly controlled in the range of 7% to 9.2%. His renal function deteriorated steadily, with eGFR declining by 2 mL/min/1.73 m2 per year. Control of RA was also poor with a DAS28-ESR of 4.46, HAQ of 0.375 and SDAI of 8.6; therefore treatment with a biologic agent was initiated when he was at 68 years of age.
At that time, the patient was 166 cm tall and weighed 73.4 kg, with a blood pressure of 136/72 mmHg, pulse rate of 72 beats per minute and a temperature of 36.7 °C. He did not have smoking habit. Joint pain was detected at the following sites: left elbow, right hip, left knee and both the hands. In addition, he had swelling of the left knee and joints in both hands. He did not present chronic constipation and recurrent gastric ulcer. Cardiothoracic ratio was 44%. Laboratory findings were as follows: white blood cell count, 10,300/μL; red blood cell count, 4.16 × 106/μL; haemoglobin, 14.4 g/dL; platelet count, 198 × 103/μL; total protein, 6.8 g/dL; albumin, 3.5 g/dL; serum urea nitrogen, 35 mg/dL; serum creatinine, 1.9 mg/dL; total cholesterol, 265 mg/dL; erythrocyte sedimentation rate (ESR), 49 mm; CRP, 0.6 mg/dL; TSH, 3.245 μU/mL; free T4 0.94 ng/dL; anti-nuclear antibody (ANA), negative; rheumatoid factor, 41 U/mL; matrix metalloprotease-3 (MMP-3), 376.3 μg/mL; and anti-cyclic citrullinated peptide antibody, >5000 U/mL. In addition, his 24-h protein excretion was 0.04 g, 24-h albumin excretion was 22 mg/dl, and estimated glomerular filtration rate (eGFR) was 29 ml/min/1.73 m2 (). There was no diabetic retinopathy. Stage IV DKD (eGRF <30 ml/min/1.73 m2) was diagnosed according to Haneda’s classification. Moreover, there were no findings of amyloidosis including hypothyroidism, chronic constipation, recurrent gastric ulcer, bradycardia and cardiothoracic ratio expansion.
Table 1. Laboratory findings.
Clinical course after initiation of tocilizumab
Tocilizumab was started at a monthly dose of 400 mg (8 mg/kg), and control of RA improved, with DAS-ESR decreasing to <1.0 and CRP falling to 0.0 mg/dL. HbA1c was also better controlled at 7% to 9%. Interestingly, eGFR did not deteriorate and remained stable at around 30 ml/min/1.73 m2 after eight years of tocilizumab treatment. Furthermore, urinary protein excretion did not increase (). Blood pressure was maintained in a normal range without antihypertensive drugs.
Discussion
DKD is the major cause of ESRD requiring haemodialysis. Serum IL-6 levels are elevated in patients with diabetes, and hyperglycaemia also increases the serum IL-6 level [Citation6] while IL-6 has a role in microvascular complications of diabetes. A comparison between 97 women with T2DM and 107 women without T2DM suggested that inflammatory markers were higher in the T2DM group, including IL-6, CRP, fibrinogen and the leucocyte count [Citation7]. Also, a study of 69 patients with type 1 diabetes suggested that the IL-6 concentration was correlated with the fasting plasma glucose level, lipid profile and body mass index, and that these correlations were stronger in the patients who had microvascular complications [Citation8]. Furthermore, the serum level of IL-6 was reported to be higher in T2DM patients with nephropathy than in those without nephropathy. Moreover, there is a relationship between the severity of diabetic glomerulopathy, including mesangial expansion, and expression of IL-6 mRNA in glomerular cells [Citation4].
IL-6 plays an important role in the progression of renal disease by promoting the proliferation of podocytes and mesangial cells, inducing endothelial cell dysfunction that leads to vasoconstriction, and causing tubular atrophy [Citation5]. It has also been reported that IL-6 is associated with microalbuminuria and with the width of glomerular basement membrane [Citation9].
Immunosuppressive therapy may prevent the progression of diabetic nephropathy. Sumida et al. reported that biologic agents could lower the risk of incident CKD [Citation10]. Infliximab decreases urinary albumin excretion in diabetic rats [Citation11]. In addition, mycophenolate mofetil prevents albuminuria and glomerulosclerosis without affecting blood pressure or blood glucose in a rat model [Citation12].
IL-6 receptor blockade may also prevent the progression of diabetic nephropathy. It was reported that IL-6 induced apoptosis of podocytes in rats, while an IL-6 neutralizing antibody blocked podocyte apoptosis [Citation5], and another study showed that blocking of IL-6 signaling reduced glucose levels and podocyte damage in a rat model [Citation13]. However, the effect of IL-6 receptor blockade on diabetic nephropathy has not been reported.
Tocilizumab blocks signalling via the IL-6 receptor and is effective for various inflammatory diseases, including Castleman’s disease with AA amyloidosis and systemic rheumatoid vasculitis with necrotizing crescentic glomerulonephritis [Citation14–16]. Nishimoto et al. [Citation17] reported that Tocilizumab could improve the symptoms and biochemical abnormalities associated with Castleman’s disease. Okuda et al. [Citation18] reported that Patients with AA amyloidosis responded to Tocilizumab. The patient with RA sometimes presents AA amyloidosis. However, though histological proof was not conducted, clinical symptoms related to amyloidosis were lacking in this patient. The clinical course of our patient suggested that tocilizumab also has the potential to be used to treat DKD.
In conclusion, our patient’s RA responded to tocilizumab and his renal functions also remained stable for 8 years, even though glycaemic control was not so good. Considering the potential role of IL-6 in DKD, tocilizumab may prevent the progression of renal dysfunction even in patients with stage IV DKD.
Statement of Ethics and Patient Consent
The present report adhered to the Declaration of Helsinki. The authors confirm that I have obtained written consent from the patient.
Conflict of interest
None
References
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045.
- Pavkov ME, Knowler WC, Bennett PH, et al. Increasing incidence of proteinuria and declining incidence of end-stage renal disease in diabetic Pima Indians. Kidney Int. 2006;70:1840–1846.
- de Zeeuw D, Akizawa T, Audhya P, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–2503.
- Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442.
- Su H, Lei CT, Zhang C. Interleukin-6 signaling pathway and its role in kidney disease: an update. Front Immunol. 2017;8:405.
- Dotson S, Freeman R, Failing HJ, et al. Hypoglycemia increases serum interleukin-6 levels in healthy men and women. Diabetes Care. 2008;31:1222–1223.
- Malenica M, Silar M, Dujic T, et al. Importance of inflammatory markers and IL-6 for diagnosis and follow up of patients with type 2 diabetes mellitus. Med Glas (Zenica). 2017;14:169–175.
- Wegner M, Araszkiewicz A, Piorunska-Stolzmann M, et al. Association between IL-6 concentration and diabetes-related variables in DM1 patients with and without microvascular complications. Inflammation. 2013;36:723–728.
- Dalla Vestra M. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16:S78–S82.
- Sumida K, Molnar MZ, Potukuchi PK, et al. Treatment of rheumatoid arthritis with biologic agents lowers the risk of incident chronic kidney disease. Kidney Int. 2018;93:1207–1216.
- Moriwaki Y, Inokuchi T, Yamamoto A, et al. Effect of TNF-alpha inhibition on urinary albumin excretion in experimental diabetic rats. Acta Diabetol. 2007;44:215–218.
- Utimura R, Fujihara CK, Mattar AL, et al. Mycophenolate mofetil prevents the development of glomerular injury in experimental diabetes. Kidney Int. 2003;63:209–216.
- Lei CT, Su H, Ye C, et al. The classic signalling and trans-signalling of interleukin-6 are both injurious in podocyte under high glucose exposure. J Cell Mol Med. 2017;22:251–260.
- Oshima Y, Hoshino J, Suwabe T, et al. Multicentric Castleman's disease associated with IgA vasculitis (Henoch-Schonlein purpura) responding well to tocilizumab: a case report. Clin Rheumatol. 2017;36:729–733.
- Iijima T, Hoshino J, Suwabe T, et al. Tocilizumab for AA amyloidosis after treatment of multicentric Castleman disease with steroids, chemotherapy and rituximab for over 20 years. Intern Med. 2015;54:3215–3219.
- Iijima T, Suwabe T, Sumida K, et al. Tocilizumab improves systemic rheumatoid vasculitis with necrotizing crescentic glomerulonephritis. Modern Rheumatol. 2015;25:138–142.
- Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627–2632.
- Okuda Y, Ohnishi M, Matoba K, et al. Comparison of the clinical utility of tocilizumab and anti-TNF therapy in AA amyloidosis complicating rheumatic diseases. Modern Rheumatol. 2014;24:137–143.