ABSTRACT
The legalization of cannabis has resulted in rising use, wider availability, and development of highly potent strains. Patients presenting to the emergency department with cannabis toxicity and cannabinoid hyperemesis syndrome are becoming more prevalent. We present a case of a middle-age male with heavy acute cannabis use resulting in hypoventilation, somnolence, and hypothermia successfully treated in the emergency department and medical intensive care unit with a continuous naloxone infusion. The overlapping endocannabinoid and endogenous opioid systems may explain this patient's response to therapy, and this synergistic relationship is discussed.
With over 183 million estimated routine users, cannabis continues to be the most commonly used drug worldwide [Citation1]. Over the past two decades, regular cannabis use in the United States has doubled to approximately 10% of the adult population, and has more than tripled among college students [Citation2]. The legalization of cannabis has been a factor in driving recreational use within various states. Paralleling national trends, emergency department (ED) visits related to cannabis use have risen [Citation3]. From this combination of rising use, wider availability, development of highly potent strains, patients presenting to the ED with cannabis toxicity and cannabinoid hyperemesis syndrome are becoming more prevalent [Citation4,Citation5]. This not only includes adults, but also children who have accidentally or intentionally ingested cannabis [Citation6]. There is no specific antidote for cannabis toxicity. The opioid receptor antagonist naloxone is often administered to patients with suspected opioid overdose exhibiting hypoventilation, somnolence, and bradycardia [Citation7]. We present a case of a middle-age male with heavy acute cannabis use successfully treated in the ED and medical intensive care unit (MICU) for hypoventilation, somnolence, and hypothermia with a continuous naloxone infusion. The overlapping endocannabinoid and endogenous opioid systems may explain this patient's response to therapy [Citation8,Citation9].
Case report
A 40-year-old male was brought to the ED by his partner at approximately midnight because of decreased level of consciousness. Upon arrival, he appeared somnolent and diaphoretic and immediately went to the resuscitation room. Supplemental oxygen was supplied via nasal cannula at 4 L/min. Initial vital signs were: blood pressure 134/89 mmHg, pulse rate 53 beats/min, respiratory rate 8 breaths/min, and pulse oximetry (SaO2) 98% on room air. Rapid blood glucose was 153 mg/dL. The patient could slowly answer questions, and he denied drug or alcohol use. He further denied focal weakness or pain. After the first 5 minutes in the resuscitation room, the patient no longer responded to questions. His partner stated that he bought marijuana from an acquaintance earlier in the evening, and she indicated that he had no past medical history or current prescription medications.
Physical examination revealed a lean male lying quietly with eyes closed. His skin was diaphoretic and cool, and his pupils were miotic at 1 mm. He had diminished breath sounds, shallow respirations, and bradycardia. His rectal temperature was 34.2°C (ambient temperatures that evening were 24–30 °C). The remainder of his directed physical examination was normal. Due to a clinical picture suggesting opioid overdose, he received 0.1 mg naloxone intravenously (IV) with a slight increase in his respiratory rate to 11 breaths/min, a slight increase in pupil diameter to 2 mm, and no change in his motor activity or level of consciousness. Repeat vital signs after 10 minutes were blood pressure 131/76 mmHg, pulse rate 52 beats/min, and respiratory rate 9 breaths/min. His respiratory rate declined again, and he received 0.4 mg naloxone IV, which resulted in an increase in respirations to 12 breaths/min with no other clinical changes. A non-rebreather mask was applied at an oxygen flow rate of 15 L/min in lieu of the nasal cannula.
Out of concern for possible sepsis or meningitis/encephalitis, he received ceftriaxone, vancomycin, and acyclovir, and passive external rewarming was initiated. After 60 minutes, his blood pressure remained stable, but his pulse rate declined to 40 beats/min. His respiratory rate and chest excursion declined again, and he received a naloxone infusion of 0.5 mg/hr IV. His heart rate increased to 64 beats/min, and respiratory rate stabilized at 12 breaths/min. His mentation slightly improved as well, and he confirmed that he had been smoking marijuana for several hours earlier in the evening and had also used cocaine the previous day. After he was determined to be stable, the patient was transported to the radiology department accompanied by a nurse and underwent computed tomography (CT) of the head.
A single-view chest radiograph and head CT were normal. Laboratory testing revealed a normal complete blood count, basic metabolic panel, hepatic function panel, lactic acid, calcium, magnesium, protein, and urinalysis. Venous blood gas drawn while the patient was on 4 L/min nasal cannula showed a mild respiratory acidosis consistent with hypoventilation, with PO2 52 mmHg (laboratory reference range 35–55 mmHg), 83% O2 saturation (laboratory reference range 70%–100%), PCO2 58 mmHg (laboratory reference range 35–50 mmHg), HCO3 30 mEq/L (laboratory reference range 20–28 mEq/L), and pH 7.32. A rapid qualitative urine toxicology screen for barbiturates, benzodiazepines, opiates, amphetamine, and cocaine revealed the presence of only cocaine metabolite ecognine methyl ester. Confirmatory quantitative testing for these drugs was not performed. Salicylate, acetaminophen, and ethanol were undetectable. His thyroid-stimulating hormone was 1.35 µIU/mL (laboratory reference range 0.35–3.30 µIU/mL). Lumbar puncture yielded clear cerebrospinal fluid with one white blood cell, no red blood cells, no xanthochromia, no organisms seen on Gram stain, and negative viral meningitis/encephalitis panel.
The patient was admitted to the MICU after a total of 4 hours in the ED. After 8 hours in the MICU, the treating clinician discontinued the naloxone infusion. However, the patient became somnolent, bradycardic, and exhibited diminished respiratory drive an hour later, prompting resumption of the naloxone infusion for an additional 8 hours. He was discharged the following day. A sample of the patient's initial blood collected after arrival in the ED was sent to a specialty toxicology laboratory for quantitative liquid chromatography-tandem mass spectrometry analysis of 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (11-nor-9-carboxy-Δ9-THC), a metabolite of delta-9-tetrahydrocannabinol (Δ9-THC). This returned one week later with a level of 226 ng/mL (laboratory-specific positive cutoff 5 ng/mL) [Citation10].
Discussion
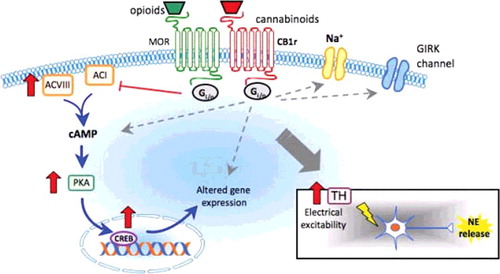
The endocannabinoid and endogenous opioid systems are syngergistic, which may explain the effect of naloxone in cannabis toxicity. Cannabinoid and opioid receptors belong to the rhodopsin subfamily of G-protein-coupled receptors which, when activated, reduce cellular levels of cyclic adenosine monophosphate (cAMP) by inhibiting adenylyl cyclase [Citation8]. Both cannabinoid and opioid receptors are located primarily at presynaptic terminals, and their activation inhibits the release of several neurotransmitters. Opioid and cannabinoid receptor activation also modifies the permeability of sodium, potassium, and calcium channels [Citation8]. Receptors of both systems coexist in the central nervous system (CNS) with overlapping distribution in the brain, brainstem, and spinal cord [Citation9]. Cannabinoid and opioid receptors also co-localize in γ-Aminobutyric acid (GABA)ergic neurons with potential coupling to a second messenger systems and formation of heterodimers between the two receptors [Citation11].
Agonists which target both systems possess shared pharmacological profiles causing sedation, hypoventilation, antinociception, hypotension, hypothermia, inhibition of intestinal motility, and drug reward-reinforcement [Citation8,Citation9]. Endocannabinoids modulate endogenous opioid function within cells from direct receptor interaction, post-receptor shared signal transduction pathways, modification of endogenous peptide release, and alteration of monoaminergic activity () [Citation8,Citation9,Citation11,Citation12]. The interplay between the endocannabinoid and endogenous opioid system is inconsistent, with the possibility for cross-agonism, cross-antagonism, cross-sensitization, and cross-tolerance depending on different ligands and concentrations. Naloxone is a μ-opioid receptor inverse agonist, and a κ- and δ-opioid receptor antagonist that has been shown to also have effects on the cannabinoid system in several animal studies [Citation13–18]. Naloxone attenuated or blocked Δ9-THC-associated mesocorticolimbic dopamine efflux in one study, suggesting a role for naloxone in altering the euphorigenic and addictive effects of both opioids and cannabis [Citation13].
Figure 1. Synergistic interactions between the endocannabinoid and endogenous opioid systems in the central nervous system.
Haney and associates investigated the interactive effects of the opioid antagonist naltrexone in heavy daily cannabis users and non-users and found paradoxical effects depending on oral versus smoking and heavy cannabis use versus non-use [Citation19–21]. Low, opioid-selective doses of naltrexone blunted low-dose Δ9-THC intoxication in chronic marijuana smokers and caused greater anxiety at high Δ9-THC doses, while in non-marijuana smokers, the opposite occurred [Citation19]. Naltrexone maintenance decreased cannabis self-administration and euphoric effects in daily cannabis smokers [Citation21]. These data demonstrate that the interaction between opioid antagonists and cannabinoid agonists varies as a function of marijuana use history. A small number of previously published case reports have documented the use of naloxone for treatment of cannabinoid overdose. Jones and colleagues reported four cases of synthetic cannabinoid toxicity presenting with somnolence and respiratory depression successfully treated with naloxone [Citation22]. Other case reports involving children with cannabis toxicity from accidental ingestion noted no response to naloxone [Citation23,Citation24]. The variable responses to opioid antagonists observed in these human studies and cases, including ours, suggests there may be a limited range of toxicity that responds to treatment. It is also important to note first responders and emergency clinicians may give up after one test dose of naloxone fails to produce an effect, however subtle. Our two initial doses of naloxone resulted in minimal changes in respiratory rate, pupil size, and mentation. Naloxone can be administered IV, or via intramuscular, subcutaneous injection, or nasal spray, and it has a half-life of one hour [Citation7,Citation14]. Similar to long-acting oral opioids, the long half-life of cannabinoids necessitates treatment of overdose with multiple and escalating doses of naloxone, or continuous infusion, as with our case [Citation14,Citation25]. Our patient also demonstrated unexpected hypothermia not explained by environmental factors, somnolence bordering on catatonia, and bradycardia. These symptoms have been documented as commonplace in studies of accidental ingestion of cannabis by animals [Citation26–28]. The mechanisms behind these symptoms likely relate to the modulation of monoaminergic transmission within the hypothalamus and downstream effects on the hypothalamic-pituitary axis and sympathetic nervous system [Citation29–33].
There may be other explanations for the profound lethargy, hypoventilation, hypothermia, and requirement for prolonged naloxone treatment exhibited by this patient. It is possible he may have taken a synthetic opioid not detected by our laboratory's qualitative drug screen, or a synthetic cannabinoid not detected by the external laboratory's assay for 11-nor-9-carboxy-Δ9-THC, which is a phytocannabinoid metabolite. Delta-9-tetrahydrocannabinol undergoes hepatic metabolization by cytochrome P450 complex-mediated microsomal hydroxylation and oxidation [Citation34]. The majority of cannabis is excreted within five days as hydroxylated and carboxylated metabolites in feces and urine, and it is difficult to ascertain acute versus chronic cannabis use from quantitative plasma metabolite analysis [Citation34]. For example, smoking an average-size marijuana cigarette containing 3.58% Δ9-THC resulted in an average peak plasma 11-nor-9-carboxy-Δ9-THC level of 8.3 ng/mL at 5 minutes and peak urine level of 77.3 ng/mL at 3 hours in a prospective study of occasional marijuana smokers [Citation35]. The authors concluded no accurate prediction of time of use was possible because 11-nor-9-carboxy-Δ9-THC has a half-life of 6 days. A study of post-accident and pre-employment Department of Defense urine drug screens of 39,532 military personnel for 11-nor-9-carboxy-Δ9-THC revealed a median concentration of 65 ng/mL (range 15 to 6129 ng/mL) and reached the same conclusion regarding predicting time of use [Citation36]. Our patient also admitted to prior-day cocaine use, confirmed by the presence of cocaine metabolite ecognine methyl ester. However, he exhibited no hyperadrenergic symptoms, and his cocaine use was not felt to be a factor in his clinical presentation and response to naloxone.
In summary, our case represents evidence that naloxone may play a role in the treatment of cannabinoid toxicity, which may be explained by overlap between the endocannibinoid and endogenous opioid systems. Clinicians should not expect the dramatic reversal in hypoventilation and somnolence customarily observed with naloxone treatment of opioid overdose. Multiple and escalating doses of naloxone may be required to achieve effect, and a continuous infusion represents a logical method of delivery to counter the long half-life of cannabinoids. Further research into the use of naloxone for acute cannabinoid intoxication may offer insight into the varied human responses observed in prior controlled studies and case reports.
Disclosure statement
The authors have no conflict of interest to report.
References
- United Nations Office on Drugs and Crime (UNODC). World Drug Report; 2017. Available from: https://www.unodc.org/wdr2017/field/WDR17_Fact_sheet.pdf
- Azofeifa A, Mattson ME, Schauer G, et al. National estimates of marijuana use and related indicators — national survey on drug use and health, United States, 2002–2014. MMWR Surveill Summ. 2016;65:1–25.
- Substance Abuse and Mental Health Service Administration. Highlights of the 2011 Drug Abuse Warning Network (DAWN) findings on drug-related emergency department visits. Available from: www.samhsa.gov/data/sites/default/files/DAWN127/DAWN127/sr127-DAWN-highlights.pdf
- Wilkinson ST, Yarnell S, Radhakrishnan R, et al. Marijuana legalization: impact on physicians and public health. Annu Rev Med. 2016;67:453–466.
- Kim HS, Anderson JD, Saghafi O, et al. Cyclic vomiting presentations following marijuana liberalization in Colorado. Acad Emerg Med. 2015;22:694–699.
- Wang GS. Pediatric concerns due to expanded cannabis use: unintended consequences of legalization. J Med Toxicol. 2017;13:99–105.
- Jordan MR, Morrisonponce D. Naloxone. 2017 Jul 1. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2017 Jun-. Available at: http://www.ncbi.nlm.nih.gov/books/NBK441910
- Robledo P, Berrendero F, Ozaita A, et al. Advances in the field of cannabinoid–opioid cross-talk. Addict Biol. 2008;13:213–224.
- Scavone JL, Sterling RC, Van Bockstaele EJ. Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal. Neurosci. 2013;248:637–654.
- ARUP Laboratories. THC Metabolite, Urine, Quantitative. Available at: http://ltd.aruplab.com/Tests/Pub/0090369
- Pickel VM, Chan J, Kash TL, et al. Compartment-specific localization of cannabinoid 1 (CB1) and mu opioid receptors in rat nucleus accumbens. Neurosci. 2004;127:101–112.
- Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–395.
- Chen JP, Paredes W, Li J, et al. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl). 1990;102:156–162.
- Sirohi S, Dighe SV, Madia PA, et al. The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity. J Pharmacol Exp Ther. 2009;330:513–519.
- Justinova Z, Tanda G, Munzar P, et al. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology. 2004;173:186–194.
- Braida D, Pozzi M, Cavallini R, et al. Conditioned place preference induced by the cannabinoid agonist CP 55, 940: interaction with the opioid system. Neuroscience. 2001;104:923–926.
- Navarro M, Carrera MR, Fratta W, et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–5350.
- Braida D, Iosuè S, Pegorini S, et al. Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol. 2004;506:63–69.
- Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–1403.
- Cooper ZD, Haney M. Opioid antagonism enhances marijuana's effects in heavy marijuana smokers. Psychopharmacology. 2010;211:141–148.
- Haney M, Ramesh D, Glass A, et al. Naltrexone maintenance decreases cannabis self-administration and subjective effects in daily cannabis smokers. Neuropsychopharmacology. 2015;40:2489–2498.
- Jones JD, Nolan ML, Daver R, et al. Can naloxone be used to treat synthetic cannabinoid overdose? Biol Psychiatry. 2017;81:e51–e52.
- Lacroix J, Farrell CA, Gaudreault P, et al. Intoxication orale au cannabis chez sept enfants [Intoxication from accidental ingestion of hashish: analysis of eight cases]. Réanimation Urgences. 1992;1:906–909.
- Carstairs SD, Fujinaka MK, Keeney GE, et al. Prolonged coma in a child due to hashish ingestion with quantitation of THC metabolites in urine. J Emerg Med. 2011;41:e69–71.
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327–360.
- Fitzgerald KT, Bronstein AC, Newquist KL. Marijuana poisoning. Top Companion Anim Med. 2013;28:8–12.
- Williams K, Wells RJ, McLean MK. Suspected synthetic cannabinoid toxicosis in a dog. J Vet Emerg Crit Care (San Antonio). 2015;25:739–744.
- Thompson GR, Rosenkrantz H, Schaeppi UH, et al. Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys. Toxicol Appl Pharmacol. 1973;25:363–372.
- Fitton AG, Pertwee RG. Changes in body temperature and oxygen consumption rate of conscious mice produced by intrahypothalamic and intracerebroventricular injections of delta 9-tetrahydrocannabinol. Br J Pharmacol. 1982;75:409–414.
- Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, Lichtman AH. Delta9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J Pharmacol Exp Ther. 2005;314:329–337.
- Singh PP, Das PK. Role of catecholamines in the hypothermic activity of cannabis in albino rats. Psychopharmacology. 1976;50:199–204.
- Smiley KA, Karler R, Turkanis SA. Effects of cannabinoids on the perfused rat heart. Res Commun Chem Pathol Pharmacol. 1976;14:659–675.
- Borgen LA, Lott GC, Davis WM. Cannabis-induced hypothermia: a dose-effect comparison of crude marihuana extract and synthetic 9 -tetrahydrocannabinol in male and female rats. Res Commun Chem Pathol Pharmacol. 1973;5:621–626.
- Sharma P, Murthy P, Bharath MM. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry. 2012;7:149–156.
- Manno JE, Manno BR, Kemp PM, et al. Temporal indication of marijuana use can be estimated from plasma and urine concentrations of delta9-tetrahydrocannabinol, 11-hydroxy-delta9-tetrahydrocannabinol, and 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid. J Anal Toxicol. 2001;25:538–549.
- Jemionek JF, Copley CL, Smith ML, et al. Concentration distribution of the marijuana metabolite Delta9-tetrahydrocannabinol-9-carboxylic acid and the cocaine metabolite benzoylecgonine in the department of defense urine drug-testing program. J Anal Toxicol. 2008;32:408–416.
