ABSTRACT
Brake fluid frequently contains diethylene glycol, a poison known to cause kidney failure, metabolic acidosis, coma and death. No consensus exists regarding management of accidental pediatric brake fluid exposures. We sought to better characterize small-volume brake fluid exposures in children less than 6 years old. We retrospectively reviewed a single poison center's database from 2000 to 2014 for all small-volume, oral, unintentional exposures to brake fluid in children less than 6 years of age. We included cases followed to a known outcome. In all cases, we attempted to confirm the clinical outcome via telephone call between October and December of 2014. Data collected included gender, age, month and year of exposure, caller site, exposure site, management site, National Poison Data System (NPDS) medical outcome, estimated quantity of brake fluid ingested, clinical effects, therapies, and laboratory data. Initial database query yielded 121 cases. We excluded 69 cases, leaving 52 for analysis. Forty cases resulted in no effect; 12 resulted in a minor effect. No cases resulted in a moderate effect, major effect, or death. These retrospective observational data suggest acute exposures to small or unknown amounts of brake fluid in children result in minor effects. These children likely can be managed expectantly.
Introduction
Brake fluid is a hydraulic fluid used to transfer and amplify braking force in motorized vehicles [Citation1]. Most brake fluids in North America are classified by the U.S. Department of Transportation, three of which (DOT 3, DOT 4, DOT 5.1) contain glycol ethers, including diethylene glycol (DEG).
DEG (CAS #111-46-6) is a poison responsible for multiple mass-poisonings throughout modern history [Citation2–4]. Its use as a diluent for sulfanilamide resulted in the deaths of 105 people in 1937 and prompted the U.S. Congress to pass the Federal Food, Drug and Cosmetic Act in 1938, which greatly increased the power of the U.S. Food and Drug Administration to oversee the safety of medications [Citation5]. Severe DEG toxicity can result in an anion gap metabolic acidosis, renal failure, seizures, central nervous system (CNS) depression, coma, and death [Citation6]. Unlike ethylene glycol, DEG concentrations are rarely measurable in real time by a clinical laboratory. Furthermore, approaches to treatment for DEG poisoning vary and can range from careful observation to antidotal therapy with fomepizole and hemodialysis depending on practitioner preference and severity of toxicity [Citation7].
Concentrations of DEG in brake fluids vary tremendously, even within the same product. For instance, Prestone® Hi-Temp Brake Fluid contains between 0% and 60% DEG, with varying concentrations of more than a dozen additional glycol ethers [Citation8]. In contrast, STP® Brake Fluid contains between 10% and 20% DEG [Citation9], while Valvoline™ DOT 3 Brake Fluid contains between 10% and 15% DEG [Citation10]. The following formula estimates the serum concentration of an alcohol:
Conservatively assuming one mouthful equals 5 mL [Citation11], if a 10-kg child ingests one mouthful of 60% DEG brake fluid, the estimated concentration would be ((5 mL × 0.6 × 1.12)/(1 L/kg × 10 kg)) × 100 = 36 mg/dL. For perspective, the threshold concentration for treatment of ethylene glycol poisoning is 20 mg/dL [Citation7]. Unlike ethylene glycol, however, DEG has no consensus treatment threshold.
Complicating matters is the fact that brake fluid is generally a heterogeneous collection of many other glycol ethers in addition to DEG. While a “worst-case” DEG concentration could be calculated, this still paints an incomplete picture of the possible toxicity to the potentially poisoned child, as ingestions of brake fluid containing other glycol ethers have resulted in a similar clinical picture (anion gap metabolic acidosis and coma) [Citation12]. Nevertheless, the material safety data sheets (MSDS) for most brake fluids reveal that DEG has the lowest exposure limit of the numerous glycol ethers, suggesting it is the most toxic constituent and thus the focus of most clinical decisions [Citation8].
An uncertain toxicity threshold, variable DEG concentrations in brake fluids, inability to obtain timely serum DEG concentrations, and lack of consensus on treatment regimens make management of accidental pediatric brake fluid exposures particularly difficult. Unintentional pediatric brake fluid exposures are common; in 2015, US poison centers reported 883 isolated exposures to brake fluid, 239 of which were in children less than 6 years of age [Citation13]. We therefore sought to better characterize single-substance small-volume brake fluid exposures in children less than 6 years old to more fully understand this clinical conundrum.
Methods
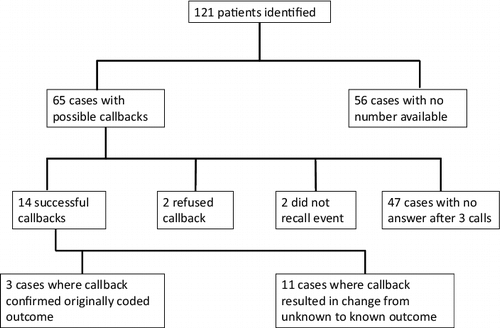
We conducted a retrospective observational case series approved by our Human Subjects Research Committee (HSR #15–3971). We searched our poison center's electronic database (Toxicall®, Computer Automated Systems, Aurora, CO) from January 2000 through October 2014 using the Micromedex® codes for “automotive product: brake fluid (0201001)” and “automotive product: other glycol (0051220).” We reviewed these cases for accurate product coding. Our case definition included all children less than 6 years of age exposed to brake fluid whose exposure amount was less than or equal to one of the following: 5 mL, 1 taste/lick/drop, 1 sip, 1 tablespoon, 3 teaspoons, 1 fluid ounce, or unknown. We followed all identified cases, when possible, with a phone call between October and December of 2014 to confirm or determine a known outcome as part of an institutional quality assurance project. depicts our standardized questions. We defined determination or confirmation of a known outcome as an a priori requisite for inclusion in the final analysis. We made three attempts to contact each caller before follow-up was deemed lost. We excluded isolated ocular and dermal exposures and exposures without a known outcome from final analysis.
Two data abstractors (JBC, SJS) abstracted each case. Both abstractors are board-certified medical toxicologists. Data collected included gender, age, month and year of exposure, caller site, exposure site, management site, medical outcome, quantity, clinical effects, therapies, if transfer to a tertiary center occurred, and laboratory data including serum bicarbonate, anion gap, serum osmolality, osmol gap, and any measured glycol concentrations. Final outcomes were “no effect,” “minor effect,” “moderate effect,” “major effect,” or “death” according to American Association of Poison Control Centers (AAPCC) standardized criteria [Citation13].
We entered all data into a standardized spreadsheet (Microsoft Excel 2011, Redmond, WA). Both abstractors reviewed free-text case notes to corroborate all coded data. Disagreement occurred on 10 cases between abstractors; in all cases, disagreement occurred due to clerical errors that were subsequently corrected.
Results
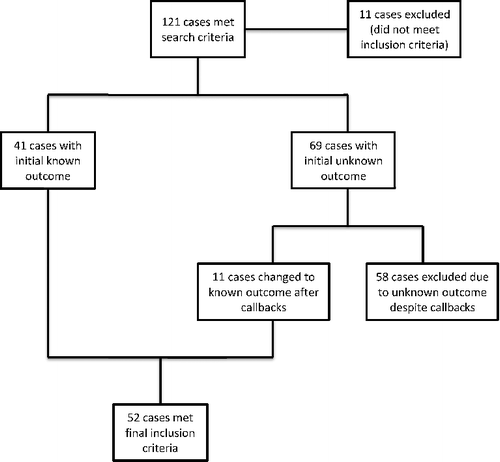
There were 121 cases meeting inclusion criteria upon initial query of our database. After all possible phone follow-ups were completed, we excluded 69 cases. Eleven excluded cases did not meet inclusion criteria upon review of the case notes; two were confirmed to be propylene glycol only, two consumed more than one mouthful, two were confirmed not to be glycol ingestions, four were isolated ocular or dermal exposures, and one was confirmed to be transmission fluid rather than brake fluid. We excluded 58 additional cases lacking known outcomes following all callbacks. They were coded as follows: nine as “not followed (judged as non-toxic),” 41 as “not followed (minimal clinical effects possible),” five as “unable to follow (potentially toxic),” and three as “unrelated effect (not responsible).”
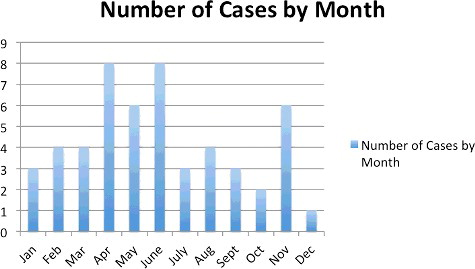
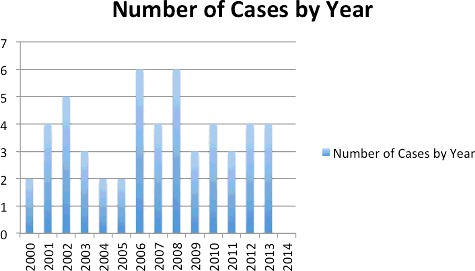
Fifty-two cases met final inclusion criteria. Forty-one cases had known outcomes prior to callbacks. In 11 cases, callbacks resulted in a change from an unknown to a known outcome. shows the results of callbacks; shows all cases meeting final inclusion criteria. and describe the monthly and annual distribution of cases. describes the demographic data.
Table 1. Demographic data.
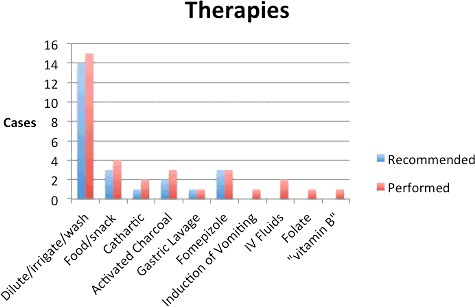
There were 40 cases with no reported effect; 12 cases resulted in a minor effect. No cases had an outcome coded as moderate effect, major effect, or death. Of the 12 cases with symptoms, three had oral irritation, three had nausea, five had vomiting, and four had coughing or choking. One child sprayed in the face with brake fluid had pain and irritation of the skin of the face as well as conjunctivitis; all symptoms resolved with irrigation and supportive care at home. One patient had drowsiness and was described by the treating physician as having “eyes rolling back in his head” on arrival but resolved upon placement of a peripheral intravenous line. This patient's arterial pH was 7.35 with a pCO2 of 41 and an osmol gap of 7. Serial metabolic panels were normal, although exact values were not recorded. Twenty-three cases were managed at home. In cases in which the initial call was from a health care facility, nine were treated, evaluated and released; four were admitted to a non-critical care unit. Among patients referred to a health care facility by the poison center, nine were treated, evaluated and released while seven were admitted to a non-critical care unit. Four patients transferred to a tertiary care center; all were treated and released. Therapies ranged from expectant management and supportive care to vitamins, gastrointestinal decontamination and fomepizole. In total, 25 patients received some form of therapy. lists the therapies used.
Laboratory data were sparse and poorly recorded. Four patients had ethylene glycol concentrations recorded, all of which were undetectable. No patients had a DEG concentration recorded. Twelve cases had a recorded osmol gap; all were “normal” with the exception of one patient who had an osmol gap of −20 with a serum osmolality of 269 mmol/kg. Twenty-six patients had a basic chemistry panel recorded. In our institution, this typically includes a serum sodium, potassium, bicarbonate, chloride, calcium, glucose, and creatinine. Twenty-five patients had chemistry panels recorded as within normal limits. One patient had a chemistry panel notable for a non-anion gap metabolic acidosis. This initial bicarbonate was 17 mEq/L with an anion gap of 13 and a creatinine level of 0.42 mg/dL. A repeat chemistry panel 4 hours later revealed no changes except a rise in bicarbonate to 19 mEq/L. The child had no symptoms and was discharged home.
Discussion
Brake fluid is a heterogeneous collection of various substances, some of which are more toxic than others. DEG is generally the most toxic component of brake fluid, and the paucity of evidence surrounding the natural history of brake fluid ingestion presents a unique management challenge. DEG is colorless, odorless, and hygroscopic, with a melting point of −6.5 °C and a boiling point of 245 °C [Citation6]. It has a sweet taste, making it potentially appealing to children. Like the toxic alcohols methanol and ethylene glycol, the metabolites of DEG (and not DEG itself) are responsible for the majority of its toxicity. As with other toxic alcohols, however, the parent compound causes altered mental status [Citation14].
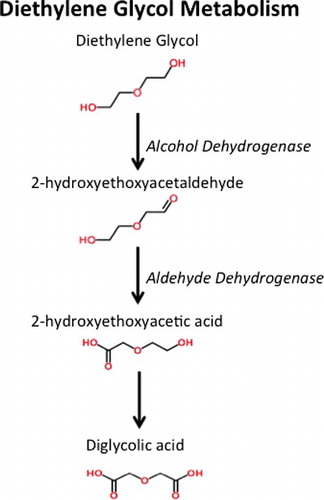
Like other toxic alcohols, DEG undergoes metabolism by both alcohol dehydrogenase and aldehyde dehydrogenase () to form 2-hydroxyethoxyacetic acid (HEAA) [Citation6,Citation15] and diglycolic acid (DGA). While HEAA appears to be responsible for the acidosis in DEG poisoning, DGA is the metabolite responsible for kidney injury [Citation14].
Our findings demonstrate that consensus does not exist regarding management of accidental pediatric brake fluid ingestions of small or unknown volumes. In our series, management ranged from home observation to admission, gastric decontamination, fomepizole administration and serial lab testing to assess for renal failure and acidosis. Cases of accidental DEG or brake fluid poisoning in children are not well documented, contributing to confusion and a lack of standardized management. Brophy et al. documented the first DEG poisoning in a child with a confirmed DEG serum concentration, in 2000 [Citation16]. This 10-kg, 17-month-old girl was found drooling and coughing next to an open bottle containing DEG. She was transported to a tertiary care center and received empiric dialysis and fomepizole. Her pre-dialysis serum DEG concentration, later obtained after her clinical course was complete, returned at 1.7 mg/dL. A post-dialysis DEG concentration was undetectable. The authors concluded that administration of fomepizole likely prevented formation of toxic metabolites, and that aggressive hemodialysis clears DEG. Evidence suggests that fomepizole prevents the formation of both HEAA and DGA [Citation17], and this case along with the physical properties of DEG suggests that hemodialysis represents a reasonable and effective therapy in the severely poisoned patient. Acute pediatric hemodialysis, however, is not readily available in most centers, and carries significant risks including bleeding and infection [Citation18]. In addition, fomepizole is not readily available in all emergency departments, particularly in rural environments [Citation19]. It is expensive; the average wholesale price as of November 2017 is $1290 for 1.5 mL of a 1 g/mL vial [Citation20]. Furthermore, at least one case of anaphylaxis to fomepizole has been documented [Citation21], and fomepizole has been intermittently on shortage [Citation22]. The use of fomepizole in this fashion is also off-label.
It is also unclear if a serum DEG concentration of 1.7 mg/dL requires treatment at all. Even in ethylene glycol and methanol poisoning, for which serum concentrations are often readily available, the treatment threshold of 20 or 25 mg/dL depends more on consensus than empirical data [Citation23], and authors have questioned the rationale for this low treatment threshold [Citation24]. A patient intentionally ingested a DEG-containing product in a suicide attempt and had a peak concentration of 89 mg/dL [Citation25]. He received hemodialysis and fomepizole until his DEG concentration reached 20 mg/dL. His serum chemistries over the next 24 hours indicated neither acidosis nor renal failure, and he recovered without sequelae. All of the above suggest that from both patient safety and resource utilization standpoints, defining acute treatment and hospital referral thresholds for DEG and brake fluid exposures is important.
The five patients excluded from our study that were coded as "unable to follow, judged as potentially toxic," warrant discussion. All five cases were ingestions of unknown quantities of brake fluid in children aged 2–4 years. All five were asymptomatic, and in two cases, the parents later felt the children were not actually exposed. In one callback for study purposes, the parent did not remember the call to the poison center, but noted their child never suffered from any kidney problems. Though these cases were coded by our poison center in real time to be potentially toxic, the consensus of the investigators is that these five cases do not represent high-risk exposures. Because we were unable to determine the outcome in all five cases, we excluded them in our final analysis.
Though the minimal effects observed in our series are reassuring, this study is not without limitations. Poison center documentation may have been incomplete, leading to an underrepresentation of clinical effects. Furthermore, the number of cases in our series limits our ability to exclude the potential for deaths or moderate/major effects. Based on our data, a 95% confidence interval around the reported moderate effects, major effects, or deaths in this series (n = 0) is 0%–6.7%. The outer limit of this confidence interval, of course, would not be an acceptable “miss rate.” Additionally, the so-called “non-ingestion” problem further limits interpretation of our data. As elegantly demonstrated by Lévy et al. in a retrospective review of 102 children who were exposed to toxic alcohols (in this case, methanol or ethylene glycol), only 21 out of 102 children actually had a detectable toxic alcohol concentration [Citation26]. It is reasonable to believe the same phenomenon existed in our patient population, and that a significant number of our patients had no exposure to a detectable amount of DEG or brake fluid. If this were true, our data may underestimate the severity of true exposures and the incidence of clinically significant outcomes. However, a recent abstract reviewed unintentional DEG exposures reported to the National Poison Data System (NPDS) [Citation27]. Among 12,628 DEG exposures, 3077 of which were in children under 6, only three children had a major outcome reported. All three children were under two years of age, and all three had altered mental status. One child was intubated, and no deaths occurred. Thus, even with a very high rate of non-exposure, this large cohort of single, acute, unintentional exposures suggests that the risk of a major clinical effect or death is extremely small. Because our poison center contributes to the NPDS, there is some duplication of cases in this data set. However, our data characterize cases followed to known outcomes from a single poison center where we have access to case notes (and not simply categorical NPDS data). Our data thus allow for more robust analysis than this NPDS review.
Altered mental status in the setting of brake fluid exposure likely portends more serious outcomes, as demonstrated by both NPDS data and a case reported by Wang et al. of a 22-month-old child who ingested an unknown amount of brake fluid (up to 330 mL). The child became obtunded and developed an anion gap metabolic acidosis following exposure [Citation12]. The child underwent intubation, hemodialysis and vasopressor therapy but survived with no sequelae. None of the patients in our series experienced altered mentation or required any of these advanced interventions.
Conclusion
This retrospective observational case series suggests that acute exposures to small or unknown amounts of brake fluid in children under 6 years of age result primarily in minor effects such as localized mucosal irritation and vomiting. Data from other sources suggest that children exposed to brake fluid who develop altered mental status are at higher risk. Our data, when interpreted alongside additional available data, suggest that children under 6 years of age with unknown or small-volume exposures and no alteration in mentation can be managed expectantly. Prospective data are necessary to validate this approach.
Acknowledegments
We wish to acknowlegde Deborah L. Anderson, PharmD, Director of the Minneosta Poison Control System, for her assistance with this project, as well as the staff of the Minnesota Poison Control System who care for all of our patients each and every day.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Motor vehicle brake fluids. Code of Federal Regulations, Title 49, Part 571, Section 116; Motor vehicle brake fluid.
- Wax PM. It's happening again–another diethylene glycol mass poisoning. J Toxicol Clin Toxicol. 1996;34(5):517–520.
- Centers for Disease Control and Prevention.Fatal poisoning among young children from diethylene glycol-contaminated acetaminophen — Nigeria, 2008–2009, (MMWR) Morb Mortal Wkly Rep. 2009;58(48):1345–1347.
- Sosa NR, Rodriguez GM, Schier JG, et al. Clinical, laboratory, diagnostic, and histopathologic features of diethylene glycol poisoning—Panama, 2006. Ann Emerg Med. 2014;64(1):38–47.
- Wax PM. Elixers, diluents, and the passage of the 1938 Federal Food, Drug and Cosmetic Act. Ann Intern Med. 1995;122:456–461.
- Schep LJ, Slaughter RJ, Temple WA, et al. Diethylene glycol poisoning. Clin Toxicol. 2009;47:525–535.
- Brent J. Fomepizole for the treatment of pediatric ethylene glycol and diethylene glycol, butoxyethanol, and methanol poisoning. Clin Toxicol. 2010;48:401–406.
- Prestone Products Corporation. Hi-Temp Brake Fluid DOT 3 [Material safety data sheet]. 2012; [cited 2017 Nov 15]. Available from: http://s7d9.scene7.com/is/content/GenuinePartsCompany/716474pdf?$PDF$
- The Armor All/STP Products Company. STP® Brake Fluid DOT 3 [Material safety data sheet]. 2011; [cited 2017 Nov 15]. Available from: http://whatsinproducts.com/files/brands_pdf/1338945009.pdf
- Ashland. Valvoline™ DOT 3 Brake Fluid [Material safety data sheet]. 2009; [cited 2017 Nov 15]. Available from: (http://s7d9.scene7.com/is/content/GenuinePartsCompany/725997pdf?$PDF$
- Ratnapalan S, Potylitsina Y, Tan LH, et al. Measuring a toddler's mouthful. J Pediatr. 2003;142(6):729–730.
- Wang GS, Yin S, Shear B, et al. Severe poisoning after accidental pediatric ingestion of glycol ethers. Pediatrics 2012;130(4):e1026–e1029.
- Mowry JB, Spyker DA, Brooks DE, et al. 2015. Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd annual report. Clin Toxicol (Phila). 2016;54(10):924–1109.
- Landry GM, Martin S, McMartin KE. Diglycolic acid is the nephrotoxic metabolite in diethylene glycol poisoning inducing necrosis in human proximal tubule cells in vitro. Toxicol Sci. 2011;124(1):35–44.
- Robinson CN, Latimer B, Abreo F, et al. Investigating the metabolite responsible for the renal toxicity of diethylene glycol in vitro. Clin Toxicol (Phila). 2017;55(3):196–205.
- Brophy PD, Tenenbein M, Gardner J, et al. Childhood diethylene glycol poisoning treated with alcohol dehydrogenase inhibitor fomepizole and hemodialysis. Am J Kidney Dis. 2000;35(5):958–962.
- Besenhofer LM, Adegboyega PA, Bartels M, et al. Inhibition of metabolism of diethylene glycol prevents target organ toxicity in rats. Toxicol Sci. 2010;117(1):25–35.
- Napalkov P, Felici DM, Chu LK, et al. Incidence of catheter-related complications in patients with central venous or hemodialysis catheters: a health care claims database analysis. BMC Cardiovasc Disord. 2013;13:86.
- Dart RC, Borron SW, Caravati EM, et al. Expert consensus guidelines for stocking of antidotes in hospitals that provide emergency care. Ann Emerg Med. 2009;54(3):386–394.e1.
- Lexi-Comp, Inc. Lexi-drugs. Lexi-Comp; 2017.
- Keeling T, Orozco B, Cole J, et al. Anaphylaxis following fomepizole for ethylene glycol poisoning [abstract]. Clin Toxicol (Phila). 2014; 52:750.
- American Society of Hospital Pharmacists. Drug shortages (fomepizole). [cited 2015 July 7]. Available from: http://www.ashp.org/menu/DrugShortages/CurrentShortages/Bulletin.aspx?id=1173
- Wiener SW. Toxic alcohols. In: Nelson LS, Lewin NA, Howland MA, editors. Goldfrank's toxicologic emergencies. 10th ed. New York (NY): McGraw-Hill; 2015. p. 1346–1357.
- Kostic MA, Dart RC. Rethinking the toxic methanol level. J Toxicol Clin Toxicol. 2003;41(6):793–800.
- Boroughf WJ, Bassett RA, Borek HA. Diethylene glycol – a DIF-ferent type of acute ingestion? [ abstract]. Clin Toxicol (Phila). 2014;52(7):768.
- Lévy A, Bailey B, Letarte A, et al. Unproven ingestion: an unrecognized bias in toxicological case series. Clin Toxicol (Phila). 2007;45(8):946–949.
- Welker KL, Stenberg JC, Kostic MA, et al. Outcome of unintentional exposures to diethylene glycol [ abstract]. Clin Toxicol (Phila). 2014;52:800.